��Ŀ����
����ʵ��عˣ�
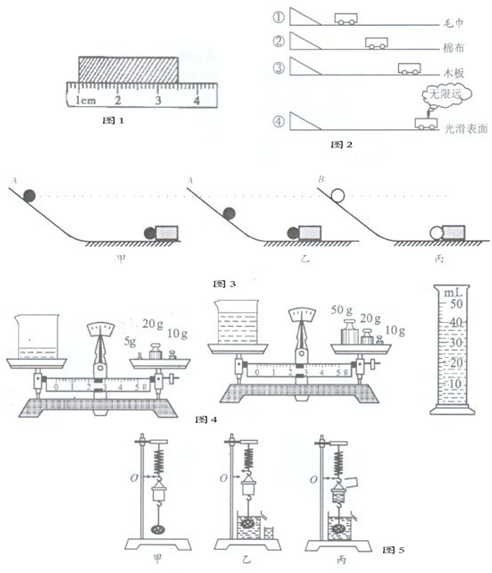
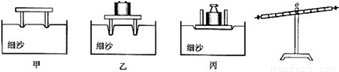
��1����ͼ����̽����ѹ��������Ч����ʲô�����йء���ʵ���У�ijͬѧ����ʵ�飮�Ƚ�ʵ��ļס�����ͼ�ɵ�ѹ��������Ч����________�йأ��Ƚ��ҡ�����ͼ�ɵ�ѹ��������Ч����________�йأ�
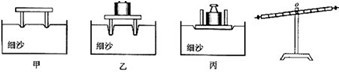
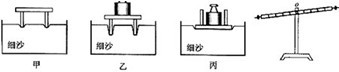
��2����ͼ�����о��ܸ�ƽ������ʱ��Ϊ��ʹ�ܸ���ˮƽλ��ƽ�⣬Ӧ�����˵�ƽ����ĸ��________��ѡ����ҡ������ڣ�Ϊ�ó�ʵ����ۣ����θı�ܸ��ܵ���������________����������õ㣬ʹ�ܸ�ʼ����________λ�ñ���ƽ�⣮
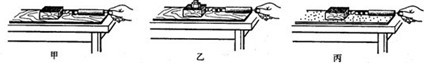
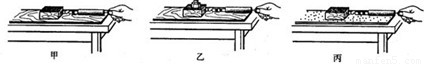
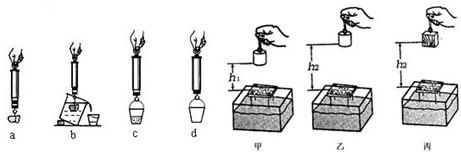
��3����̽��������Ħ�����Ĵ�С��ʲô�����йء�ʱ��С������ʵ��װ�ý���ʵ�飮��ͨ���Ƚ�ʵ���________��ͼ�ɵã��ڽӴ���ֲڳ̶�һ��ʱ������Ħ�����Ĵ�С��ѹ����С�йأ�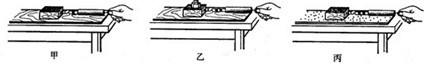
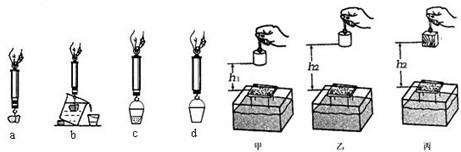
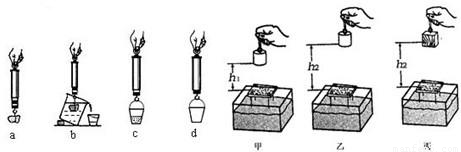
��4��С��ͬѧ��̽���������Ĵ�С����ʲô��ʱ���õ��ɲ����ơ�Сʯ�顢�ձ���СͰ�����Ľ���ʵ�飬ͼ�е�a��b��c��d ��ʵ���ĸ������ʾ��ͼ������d����������ǿ�Ͱ�ܵ���________����ʵ���������ȷ˳����________������ţ���
��5��С������װ��ϸɳ�ĺ��ӡ�С�������롢ľ������ģ�̽�����������ܵĴ�С����Щ�����йء�������̣�С����ͨ���۲�ʲô���ж��������ܴ�С�ģ�
�⣺��1��������ͼ���������ͬ��ѹ����ͬ�������о�ѹ������Ч����ѹ����С�Ĺ�ϵ���ұ���ͼѹ���Ĵ�С��ͬ�����������ͬ������̽��ѹ������Ч������������Ĺ�ϵ��
��2����ͼ֪���ܸ˵��Ҷ˸ߣ�����Ӧ��ƽ����ĸ�����ƶ���ʹ�ܸ���ˮƽλ��ƽ�⣮Ϊ�ó�ʵ����ۣ�Ӧ��θı�ܸ��������Ĵ�С����������õ㣮��ʹ�ܸ���ˮƽλ��ƽ�⣮
��3��������ͼ�Ӵ���Ĵֲڳ̶���ͬ��ѹ����С��ͬ������̽��Ħ������ѹ����С�Ĺ�ϵ��
��4����̽���������Ĵ�С����ʲô��ʱ��Ҫ�Ƚϸ�����С���ſ�ˮ������֮��Ĺ�ϵ��������Ҫ�Ȳ����Ͱ��������Сʯ���ڿ����е��������������ܹ���ȷ�ز��Сʯ���ܵ��ĸ������ſ�ˮ��������
��5����ͼ֪��С������ɳ��Խ�˵�������С�����Ĺ�Խ�࣬������е���������Խ�����Դ�ʵ���У�ͨ��С������ɳ�е�������ж��������ܵĴ�С��
�ʴ�Ϊ����1��ѹ���Ĵ�С�������������2���ң���С��ˮƽ����3���ס��ң���4��������a d b c����d a b c������5���۲�С������ɳ�е���ȣ�
��������1��ѹ��������Ч����ѹ����С����������Ĵ�С�йأ����о���ѹ��������Ч����ʲô�����йء�ʱ��Ҫ�õ����Ʊ�������
��2���ܸ˵ĵ�ƽ��������ƽ�ĵ�ƽ���ƣ��Ƕ˸�ƽ����ĸ���Ķ˵��ڣ���ʵ���У�Ϊ�˱��ڲ������ۣ�Ҫ���ָܸ���ˮƽλ��ƽ�⣮
��3������Ħ�����Ĵ�С��ѹ���ͽӴ���Ĵֲڳ̶��йأ�Ҫ�о�Ħ������ѹ����С�Ĺ�ϵ����ʹ�Ӵ���ֲڳ̶�һ�����ı�ѹ����С��
��4���������������������Ĵ�С�Ĺ��ߣ���̽���������Ĵ�С����ʲô��ʱ��Ҫ�Ƚϸ�����С���ſ�ˮ������֮��Ĺ�ϵ��Ҫ�⸡�������Ȳ�ʯ���ڿ����е����������ſ�ˮ�����������Ȳ��Ͱ��������
��5������������Ĺ�Խ�࣬˵��������е�����Խ�������ڴ�ʵ��̽�����������ܵĴ�С����Щ�����йء�ʱ��ͨ��С������ɳ�е�������ж��������ܵĴ�С��������ת������
����������ͨ����ͬ��ʵ�鿼���˿��Ʊ�������ʵ���е�Ӧ�ã���ij�����������������й�ʱ��Ҫ�ÿ��Ʊ���������̽������̽��������Ҫע��ı�����Ͳ��������
��2����ͼ֪���ܸ˵��Ҷ˸ߣ�����Ӧ��ƽ����ĸ�����ƶ���ʹ�ܸ���ˮƽλ��ƽ�⣮Ϊ�ó�ʵ����ۣ�Ӧ��θı�ܸ��������Ĵ�С����������õ㣮��ʹ�ܸ���ˮƽλ��ƽ�⣮
��3��������ͼ�Ӵ���Ĵֲڳ̶���ͬ��ѹ����С��ͬ������̽��Ħ������ѹ����С�Ĺ�ϵ��
��4����̽���������Ĵ�С����ʲô��ʱ��Ҫ�Ƚϸ�����С���ſ�ˮ������֮��Ĺ�ϵ��������Ҫ�Ȳ����Ͱ��������Сʯ���ڿ����е��������������ܹ���ȷ�ز��Сʯ���ܵ��ĸ������ſ�ˮ��������
��5����ͼ֪��С������ɳ��Խ�˵�������С�����Ĺ�Խ�࣬������е���������Խ�����Դ�ʵ���У�ͨ��С������ɳ�е�������ж��������ܵĴ�С��
�ʴ�Ϊ����1��ѹ���Ĵ�С�������������2���ң���С��ˮƽ����3���ס��ң���4��������a d b c����d a b c������5���۲�С������ɳ�е���ȣ�
��������1��ѹ��������Ч����ѹ����С����������Ĵ�С�йأ����о���ѹ��������Ч����ʲô�����йء�ʱ��Ҫ�õ����Ʊ�������
��2���ܸ˵ĵ�ƽ��������ƽ�ĵ�ƽ���ƣ��Ƕ˸�ƽ����ĸ���Ķ˵��ڣ���ʵ���У�Ϊ�˱��ڲ������ۣ�Ҫ���ָܸ���ˮƽλ��ƽ�⣮
��3������Ħ�����Ĵ�С��ѹ���ͽӴ���Ĵֲڳ̶��йأ�Ҫ�о�Ħ������ѹ����С�Ĺ�ϵ����ʹ�Ӵ���ֲڳ̶�һ�����ı�ѹ����С��
��4���������������������Ĵ�С�Ĺ��ߣ���̽���������Ĵ�С����ʲô��ʱ��Ҫ�Ƚϸ�����С���ſ�ˮ������֮��Ĺ�ϵ��Ҫ�⸡�������Ȳ�ʯ���ڿ����е����������ſ�ˮ�����������Ȳ��Ͱ��������
��5������������Ĺ�Խ�࣬˵��������е�����Խ�������ڴ�ʵ��̽�����������ܵĴ�С����Щ�����йء�ʱ��ͨ��С������ɳ�е�������ж��������ܵĴ�С��������ת������
����������ͨ����ͬ��ʵ�鿼���˿��Ʊ�������ʵ���е�Ӧ�ã���ij�����������������й�ʱ��Ҫ�ÿ��Ʊ���������̽������̽��������Ҫע��ı�����Ͳ��������

��ϰ��ϵ�д�
�����Ŀ