��Ŀ����
����Ŀ��ijʵ��С��̽������˿�����С�볤�ȵĹ�ϵ������ȡһ�δ�ϸ���ȵĽ���˿��ֱ������A��B�������ϣ��ڽ���˿�ϰ�װһ���ɻ����Ľ�����P��ʵ���һ��ṩ���������ģ���ѹ����������������飨��ѹ3V����������������20 ![]() 2A�����̶ȳߡ����غ͵������ɣ�
2A�����̶ȳߡ����غ͵������ɣ�
��1�������ñʻ��ߴ��浼�ߣ���ͼ����ʾ��ʵ���·����������Ҫ���������Ļ�ƬP�����ƶ�ʱ��������ʾ�����
��2���պϿ���ǰ���������������Ļ�Ƭ����������������ѡ����ҡ����ˣ�
��3����ʵ��С��պϿ��غ�С��ͬѧ�۲췢�ֵ�ѹ���Ķ���Ϊ3V����������ָ�뼸��û�з���ƫת�����·���ܳ��ֵĹ����ǵ���˿AP���ַ��� ��
��4��ij��ʵ���в�õ�ѹ���Ķ���Ϊ2.1V��������ָ��ƫת��ͼ����ʾ����������Ķ���ΪI=A����ʱ����˿�ĵ���R= ![]() ��
��
��5��ʵ�����ƶ�������P���ֱ���AP�εij���L�Ͷ�Ӧ�ĵ���ֵR�����������
�����������ݣ���֪ ��
��6��С��ͬѧ��Ϊ�����ڵ�Դ��ѹû�г�����ѹ����ѡ���̵����ֵ������ڱպϿ���ǰ�����Խ��������Ļ�Ƭ��������λ�ã�����Ϊ�����뷨����ѡ���ȷ�������ģ������ǣ� ��
���𰸡�
��1����:������ʾ��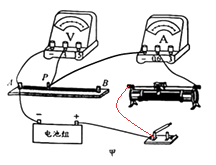 ��
��
��2����
��3����·��
��4��0.42,5
��5��������������,����ĵ����볤�ȳ����ȣ�
��6������,�������IJ���ֵ���ܳ�����ѡ���̵����ֵ��
����������1���������Ļ�ƬP�����ƶ�ʱ��������ʾ����������·�еĵ����С������Ƭ��ߵ���˿�����·�У�������ʾ��
 ;
;
��2���պϿ���ǰ���轫�����������Ļ�Ƭ�Ƶ���ֵ����λ�ã�������Ƭ��������ˣ�
��3�������������ֵ�ѹ���Ķ���Ϊ3V����������ָ�뼸��û�з���ƫת�����·���ܳ��ֵĹ����ǵ���˿AP���ַ�����·��
��4����ͼ�ҿ�֪��������������Ϊ0��0.6A���ֶ�ֵΪ0.02A��ʾ��Ϊ0.42A����![]() ��֪������˿�ĵ��裺
��֪������˿�ĵ��裺![]() ��
��
��5���ɱ���������֪�����賤������Ϊԭ���ļ���������ֵ������Ϊԭ���ļ����������ֵ�볤�ȵı�ֵ��һ��������������������һ��ʱ������ĵ����볤�ȳ����ȣ�
��6������·�����ڵ�·��������ʹ��·�еĵ��������������������̣����ջ��������������ڱպϿ���ǰ��Ҫ�ѻ����������Ļ�Ƭ���������ֵ��.
�ʴ�Ϊ����1�� ����2����3����·����4��0.42��5����5�������������䣬����ĵ����볤�ȳ����ȣ���6�����������IJ���ֵ���ܳ�����ѡ���̵����ֵ.
����2����3����·����4��0.42��5����5�������������䣬����ĵ����볤�ȳ����ȣ���6�����������IJ���ֵ���ܳ�����ѡ���̵����ֵ.
��1�����������Ҫ��ͻ�����������ʹ�÷������������
��2�����ݵ�ѧʵ��ʱ�Ի�����������Ҫ�����������
��3�����ݵ�ѹ����ʾ���͵�·�е�������������ϵ�ԭ��
��4�����ݵ�������ʾ����ŷķ���ɼ��������˿�ĵ��裻
��5�����ݱ����е����ݽ���������������
��6�����ݵ�ѧʵ���жԻ�����������Ҫ����������������.

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�