��Ŀ����
 ijʵ��С���ͬѧ����ƽ����Ͳ����ij��Һ����ܶȣ�
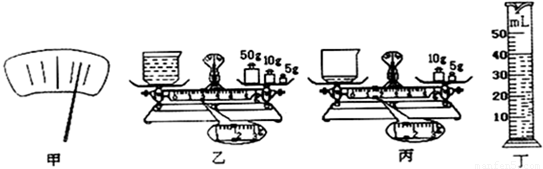
ijʵ��С���ͬѧ����ƽ����Ͳ����ij��Һ����ܶȣ���1����������ƽ����ˮƽ̨�ϣ������Ƶ���ߵ���̶ȴ������ֺ����Ҷ�ƫ�ߣ�Ӧ��ƽ����ĸ��
��2���Ȳ�����ձ�������Ϊ20g��������ձ��е���������Һ�壬������ǵ�������Ϊ49g����ʱ��ƽ����������20g������������5g������һ������ô����Ӧ���ڱ�ߵ�
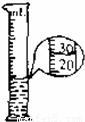
��3�����ձ��е�Һ�嵹����Ͳ�У�Һ��ﵽ��λ����ͼ��ʾ��Һ������Ϊ
��4���������ϲ������ݺͼ�������ȷ������ͬѧ���ڶ�ʵ����̼������������ʱ�������ܶȵIJ���ֵ������ʵ��ֵ
��������1��������ƽ��ƽ�⣬�۲�ָ��Ӧ����ƫ�ҵ�����ƫ������ȿ�������۲�������Ķ˸����Ķ˵���
��2��������������=���������+�����Ӧ�Ŀ̶�ֵ��
��3��Һ����ܶ��æ�=
ֱ�������
��2��������������=���������+�����Ӧ�Ŀ̶�ֵ��
��3��Һ����ܶ��æ�=
| m |
| v |
����⣺��1�������Ҷ�ƫ�ߣ�Ӧ��ƽ����ĸ���ҵ���
��2��49g=20g+20g+5g+4g���������Ӧ���ڱ�ߵ�4g����
��3����Ͳ�ķֶ�ֵ��2ml��Һ�����Ϊ30ml��ˮ������=49g-20g=29g�����ݦ�=
=
=0.97g/cm3��
��4����Һ���ܶȵĻ��������ǣ�������ƽ���Һ����ձ���������m1���ٽ�����Һ�嵹����Ͳ���������ΪV��������ƽ���ʣ��Һ����ձ�������m2��m=m1-m2
����Ȳ���ձ����������ٲ���ձ���ˮ����������ˮ������=��������ȥ�ձ�����������ô�ձ����ˮ��ȫ��������Ͳ���϶���һ����ճ���ڱ��ϣ�����������С������ܶȻ���
�ʴ�Ϊ����1���ң���2��4����3��30��0.97����4��ƫ��
��2��49g=20g+20g+5g+4g���������Ӧ���ڱ�ߵ�4g����
��3����Ͳ�ķֶ�ֵ��2ml��Һ�����Ϊ30ml��ˮ������=49g-20g=29g�����ݦ�=
| m |
| v |
| 29g |
| 30cm3 |
��4����Һ���ܶȵĻ��������ǣ�������ƽ���Һ����ձ���������m1���ٽ�����Һ�嵹����Ͳ���������ΪV��������ƽ���ʣ��Һ����ձ�������m2��m=m1-m2
����Ȳ���ձ����������ٲ���ձ���ˮ����������ˮ������=��������ȥ�ձ�����������ô�ձ����ˮ��ȫ��������Ͳ���϶���һ����ճ���ڱ��ϣ�����������С������ܶȻ���
�ʴ�Ϊ����1���ң���2��4����3��30��0.97����4��ƫ��
���������⿼������ƽ������ʱ�Ķ����Լ��ܶȵļ��㣬���漰���˲�Һ���ܶȵ�ʵ�鲽��Խ����Ӱ�죮

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ