��Ŀ����
���á������������������ʵ���У�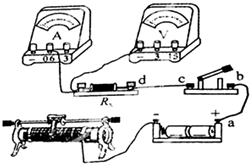
��1��ʵ��������
��2����ͼ��û����ȫ���Ӻõĵ�·ͼ�����ñʻ��ߴ��浼������������ʵ���·ͼ�����߲��ɽ��棩����������Ӧ�ĵ�·ͼ���պϿ���ǰ�����������Ļ�ƬPӦ��
��3�������������ڱ�ʵ���е������ǣ���
��4����ȷ���ӵ�·�պϿ���S������������±���
ͨ�����㷢�֣����β����Rxֵ������ͬ��������Ϊ

��1��ʵ��������
R=
| U |
| I |
R=
��| U |
| I |
��2����ͼ��û����ȫ���Ӻõĵ�·ͼ�����ñʻ��ߴ��浼������������ʵ���·ͼ�����߲��ɽ��棩����������Ӧ�ĵ�·ͼ���պϿ���ǰ�����������Ļ�ƬPӦ��
��
��
�ˣ���3�������������ڱ�ʵ���е������ǣ���
������·
������·
�����ı��·������������˵�ѹ
�ı��·������������˵�ѹ
����4����ȷ���ӵ�·�պϿ���S������������±���
| ʵ����� | �������˵ĵ�ѹU/V | �����еĵ���I/A |
| 1 | 3 | 0.3 |
| 2 | 2.1 | 0.22 |
| 3 | 1 | 0.12 |
��ѹ���������������������¶ȶԵ�����Ӱ��
��ѹ���������������������¶ȶԵ�����Ӱ��
�����������ʵ������������������
����������
�Dz���ȱ�ٵģ���������1��������������ԭ����ŷķ���ɣ�
��2���ѵ������뻬�����������������·����ѹ�������ڵ������ˣ�����ʵ���·ͼ������·ͼ���պϿ���ǰ��������������ƬӦ������ֵ���
��3������������һ������Ա�����·����һ�����Ըı��·���裬�Ӷ��ı��·������������˵�ѹ��
��4���ӵ������������¶ȶԵ����Ӱ��ȷ���������費ͬ��ԭ��Ϊ�˲����ʵ�����ݣ���·�б����л�����������
��2���ѵ������뻬�����������������·����ѹ�������ڵ������ˣ�����ʵ���·ͼ������·ͼ���պϿ���ǰ��������������ƬӦ������ֵ���
��3������������һ������Ա�����·����һ�����Ըı��·���裬�Ӷ��ı��·������������˵�ѹ��
��4���ӵ������������¶ȶԵ����Ӱ��ȷ���������費ͬ��ԭ��Ϊ�˲����ʵ�����ݣ���·�б����л�����������
����⣺��1���ɵ�·ͼ��֪���õ�ѹ�����ѹ���õ������������ʵ����÷���������裬������������ԭ����ŷķ���ɣ�����������˵�ѹU��ͨ������ĵ�������R=
�������������ֵ��
��2���ɵ�·ͼ��֪����Դ��ѹU=1.5V��2=3V�����ѹ��Ӧѡ0��3V�����̣��ѵ��������������������������·����ѹ�������ڵ������ˣ�ʵ���·ͼ��ͼ����ʾ������ʵ���·ͼ����ʵ���·ͼ��ʵ���·ͼ��ͼ����ʾ���ɵ�·ͼ��֪���պϿ���ǰ�����������Ļ�ƬPӦ����ˣ���ʱ���������������·����ֵ���
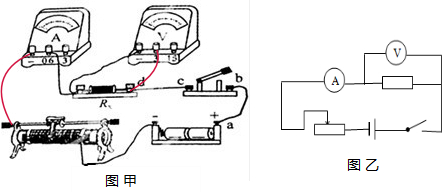
��3�����������������ڵ�·�У�һ������Ա�����·����һ�����ƶ���Ƭ���ı们�������������·����ֵ���Ӷ��ı��·�������ı�������˵�ѹ�����ж�β�����
��4�����ݱ���ʵ�����ݣ���ŷķ���ɿ�֪�����β���ĵ�����ֵ��ͬ���������ѹ������������ʹ����ĵ�����ֵ��ͬ����һ���������ֵ���¶ȵ�Ӱ�죬���¶ȱ仯���仯��Ҳ��ʹ���������ֵ��ͬ��Ϊ�������ʵ�����ݣ�ʵ���б����õ�������������
�ʴ�Ϊ����1��R=
����2��ʵ���·ͼ��ͼ����ʾ����·ͼ��ͼ����ʾ����3��������·���ı��·������������˵�ѹ����4����ѹ���������������������¶ȶԵ�����Ӱ�죻������������
| U |
| I |
��2���ɵ�·ͼ��֪����Դ��ѹU=1.5V��2=3V�����ѹ��Ӧѡ0��3V�����̣��ѵ��������������������������·����ѹ�������ڵ������ˣ�ʵ���·ͼ��ͼ����ʾ������ʵ���·ͼ����ʵ���·ͼ��ʵ���·ͼ��ͼ����ʾ���ɵ�·ͼ��֪���պϿ���ǰ�����������Ļ�ƬPӦ����ˣ���ʱ���������������·����ֵ���

��3�����������������ڵ�·�У�һ������Ա�����·����һ�����ƶ���Ƭ���ı们�������������·����ֵ���Ӷ��ı��·�������ı�������˵�ѹ�����ж�β�����
��4�����ݱ���ʵ�����ݣ���ŷķ���ɿ�֪�����β���ĵ�����ֵ��ͬ���������ѹ������������ʹ����ĵ�����ֵ��ͬ����һ���������ֵ���¶ȵ�Ӱ�죬���¶ȱ仯���仯��Ҳ��ʹ���������ֵ��ͬ��Ϊ�������ʵ�����ݣ�ʵ���б����õ�������������
�ʴ�Ϊ����1��R=
| U |
| I |
���������⿼����ʵ��ԭ��������ʵ���·ͼ������·ͼ�����������������á�ʵ���������ȣ�����ʵ��ij������⣬һ��Ҫ���գ��������ʵ���·ͼ�ṹ��������ȷ����ʵ���·ͼ��ǰ����ؼ���ʵ���·ͼҪ��ʵ���·ͼ����ϣ�

��ϰ��ϵ�д�
�����Ŀ