��Ŀ����
ijʵ��С���ͬѧ��̽��ͨ������ĵ������ѹ������Ĺ�ϵʱ����������ͼ1��ʾ�ĵ�·ͼ��ʵ��������ѡ�õĶ�ֵ������5������Դ��ѹ��3V����������������ֵ��Χ��0��15����
��1��ʵ���У�������������Ӧѡ
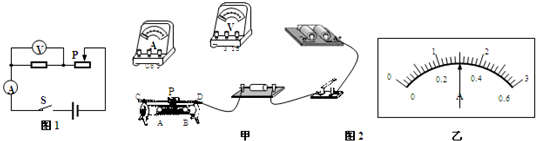
��2���ñʻ��ߴ��浼�ߣ�����ͼ2���ף���ʵ�����ĵ����ӣ�Ҫ������������Ƭ�����ƶ�ʱ�����С��
��3�����ӵ�·ʱ������Ӧ����
��4����̽���������ѹ�Ĺ�ϵʱ��ͨ�����ڱ�������ƬP��λ�����ı�
��5��Ҫ̽�����������Ĺ�ϵ����ƾͼ����ʾ�����Dz����ģ�����Ϊ���������⣩��Ӧ���ӵ�������

��1��ʵ���У�������������Ӧѡ
0.6
0.6
A����ѹ��������Ӧѡ3
3
V����2���ñʻ��ߴ��浼�ߣ�����ͼ2���ף���ʵ�����ĵ����ӣ�Ҫ������������Ƭ�����ƶ�ʱ�����С��
��3�����ӵ�·ʱ������Ӧ����
�Ͽ�
�Ͽ�
״̬�����������ӵĵ�·�������������Ļ�ƬӦ����������
��
��ѡ����ҡ����ˣ���4����̽���������ѹ�Ĺ�ϵʱ��ͨ�����ڱ�������ƬP��λ�����ı�
����
����
���˵ĵ�ѹ�����θı们Ƭλ�ã���������ѹ�͵���ֵ�������������е�һ�εĵ�����������ͼ2����ʾ�����������������У��������ݷ���ÿ�ε�ѹ������ı�ֵ����ͬ
��ͬ
�ģ�ѡ���ͬ����ͬ�������̴˿ɵó��������ѹ��ϵ�ij������ۣ�������һ��ʱ���������ѹ������
�������ѹ������
��| ʵ����� | 1 | 2 | 3 |
| ��ѹ/V | 1.5 | 2.0 | 2.4 |
| ����/A | 0.40 | 0.48 |
������ͬ��ֵ�Ķ�ֵ����
������ͬ��ֵ�Ķ�ֵ����
����������1�����ݶ�ֵ����͵�Դ��ѹ�������·�е���������ȷ�������������̣����ݵ�Դ��ѹȷ����ѹ�������̣�
��2���������붨ֵ���贮������ѹ���붨ֵ���貢�������ݻ�����������Ƭ�����ƶ�ʱ�����С��ȷ����������������ѡ��Ľ�������
��3�����ӵ�·�����У�����Ҫ�Ͽ��������������Ļ�Ƭ���������ֵ����
��4��̽���������ѹ�Ĺ�ϵʱ����Ҫ���ϸı䶨ֵ�������˵ĵ�ѹ����ͨ�����ڻ����������Ļ�Ƭʵ�ֵģ��������ѹ�Ĺ�ϵ�ǣ�����һ��ʱ���������ѹ�����ȣ�
��5��Ҫ̽�����������Ĺ�ϵ������ı䶨ֵ�������ֵ���ƶ���Ƭ���ֶ�ֵ�������˵ĵ�ѹ���䣮
��2���������붨ֵ���贮������ѹ���붨ֵ���貢�������ݻ�����������Ƭ�����ƶ�ʱ�����С��ȷ����������������ѡ��Ľ�������
��3�����ӵ�·�����У�����Ҫ�Ͽ��������������Ļ�Ƭ���������ֵ����
��4��̽���������ѹ�Ĺ�ϵʱ����Ҫ���ϸı䶨ֵ�������˵ĵ�ѹ����ͨ�����ڻ����������Ļ�Ƭʵ�ֵģ��������ѹ�Ĺ�ϵ�ǣ�����һ��ʱ���������ѹ�����ȣ�
��5��Ҫ̽�����������Ĺ�ϵ������ı䶨ֵ�������ֵ���ƶ���Ƭ���ֶ�ֵ�������˵ĵ�ѹ���䣮
����⣺��1����Դ��ѹ��3V����˵�ѹ��ѡ��0��3V���̣�ѡ�õĶ�ֵ������5������·�е���������I=
=
=0.6A����˵�����ѡ��0��0.6A���̣�
�ʴ�Ϊ��0.6��3��
��2�����������붨ֵ���贮������ѹ���붨ֵ���貢����ע�����̺�������������������������Ƭ�����ƶ�ʱ�����С������¶�ѡ������Ľ�����������ͼ��
��3����ѧʵ�飬���ӵ�·�����У�����Ҫ�Ͽ�����Ƭ�Ƶ���ֵ���������������ѡ���������Ľ���������˻�Ƭ�Ƶ��Ҷˣ�
�ʴ�Ϊ���Ͽ����ң�
��4���ٵ�̽���������ѹ�Ĺ�ϵʱ��ͨ�����ڱ�������ƬP��λ�����ı�������˵ĵ�ѹ��
�ڵ�����ѡ�����0��0.6A���̣��ֶ�ֵ��0.02A��ָ��ָ��0.2A��0.4A�м䣬�����0.3A��
�۷������ݷ������ε�ѹ������ı�ֵ
=
=
=5���ɵó��������ѹ��ϵ�ij������ۣ�����һ��ʱ���������ѹ�����ȣ�
�ʴ�Ϊ�����裻0.3����ͬ���������ѹ�����ȣ�
��4��̽�����������Ĺ�ϵ����Ҫ���Ƶ������˵ĵ�ѹ���䣬������ͬ��ֵ�ĵ��裻
�ʴ�Ϊ��������ͬ��ֵ�Ķ�ֵ���裮
| U |
| R |
| 3V |
| 5�� |
�ʴ�Ϊ��0.6��3��
��2�����������붨ֵ���贮������ѹ���붨ֵ���貢����ע�����̺�������������������������Ƭ�����ƶ�ʱ�����С������¶�ѡ������Ľ�����������ͼ��

��3����ѧʵ�飬���ӵ�·�����У�����Ҫ�Ͽ�����Ƭ�Ƶ���ֵ���������������ѡ���������Ľ���������˻�Ƭ�Ƶ��Ҷˣ�
�ʴ�Ϊ���Ͽ����ң�
��4���ٵ�̽���������ѹ�Ĺ�ϵʱ��ͨ�����ڱ�������ƬP��λ�����ı�������˵ĵ�ѹ��
�ڵ�����ѡ�����0��0.6A���̣��ֶ�ֵ��0.02A��ָ��ָ��0.2A��0.4A�м䣬�����0.3A��
�۷������ݷ������ε�ѹ������ı�ֵ
| 1.5 |
| 0.3 |
| 2 |
| 0.4 |
| 2.4 |
| 0.48 |
�ʴ�Ϊ�����裻0.3����ͬ���������ѹ�����ȣ�
��4��̽�����������Ĺ�ϵ����Ҫ���Ƶ������˵ĵ�ѹ���䣬������ͬ��ֵ�ĵ��裻
�ʴ�Ϊ��������ͬ��ֵ�Ķ�ֵ���裮
������������Ҫ������ǿ��Ʊ�����������ʵ���е����ã��Լ�ѧ����ʵ�����ݵķ����������������ܽ����������п��ij������ͣ�

��ϰ��ϵ�д�
 �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�
�����Ŀ

 �±��г�����ijʵ��С���ͬѧ��̽���������ۻ�ʱ�¶ȵı仯���ɡ�ʱ��¼��ʵ�����ݣ�
�±��г�����ijʵ��С���ͬѧ��̽���������ۻ�ʱ�¶ȵı仯���ɡ�ʱ��¼��ʵ�����ݣ� ��2012?��������һѧУijʵ��С���ͬѧ��̽��ŷķ����ʱ��������ͼ����ʾ�ĵ�·ͼ��ʵ��������ѡ�õĶ�ֵ����ֱ���4����10����20������Դ��ѹ��6V����������������ֵ��Χ��0��20����
��2012?��������һѧУijʵ��С���ͬѧ��̽��ŷķ����ʱ��������ͼ����ʾ�ĵ�·ͼ��ʵ��������ѡ�õĶ�ֵ����ֱ���4����10����20������Դ��ѹ��6V����������������ֵ��Χ��0��20���� ijʵ��С���ͬѧ��̽��ŷķ����ʱ����������ͼ��ʾ�ĵ�·ͼ��ʵ��������ѡ�õĶ�ֵ����ֱ���5����8����10������Դ��ѹ��3V����������������ֵ��Χ��0��15����
ijʵ��С���ͬѧ��̽��ŷķ����ʱ����������ͼ��ʾ�ĵ�·ͼ��ʵ��������ѡ�õĶ�ֵ����ֱ���5����8����10������Դ��ѹ��3V����������������ֵ��Χ��0��15���� ijʵ��С���ͬѧ��̽��ŷķ����ʱ����������ͼ��ʾ�ĵ�·ͼ��ʵ��������ѡ�õĶ�ֵ����ֱ���5����8����10������Դ��ѹ��3V����������������ֵ��Χ��0��15����
ijʵ��С���ͬѧ��̽��ŷķ����ʱ����������ͼ��ʾ�ĵ�·ͼ��ʵ��������ѡ�õĶ�ֵ����ֱ���5����8����10������Դ��ѹ��3V����������������ֵ��Χ��0��15����