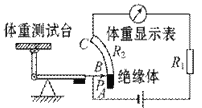
��Ŀ����
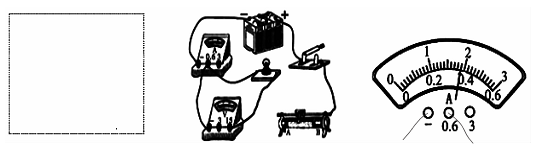
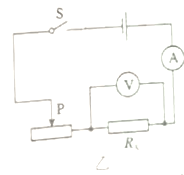
����Ŀ���÷���������裬ʵ���·��ͼ����ʾ��
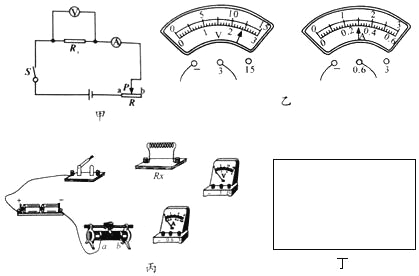
��1�����ӵ�·ʱ������Ӧ���� �ģ�
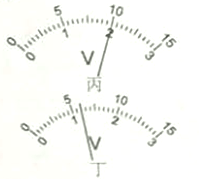
��2����ɽͬѧ��ʵ���е�ij�β�������ѹ����������Ķ�����ͼ����ʾ�����ʱ����������ֵΪ �����������ͼ�ĵ�·ͼ����ͼ�����ñʻ��ߴ��浼�߰��ɽͬѧ����ʵ��ͼ�����߲��ܽ��棩��
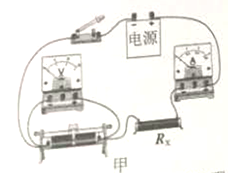
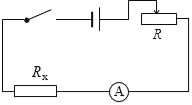
��3��ʵ���У������ѹ��ͻȻ����֪�����������������ֵΪR������������ֵΪRx����Դ��ѹ���ֲ��䣬����Դ��ѹδ֪����������ʣ�µ����IJ��Rx�������ұ߷����ڻ�����ʱ��ʵ���·ͼ���ټ�Ҫд��ʵ����̺ͼ���Rx�ı���ʽ��
a��ʵ����̣� ��
��
b��Rx�ı���ʽ��Rx= ��
���𰸡���1���Ͽ�����2��8������ͼ��ʾ����3������ͼ��ʾ��ʵ�鲽�裺������ʱ���Ƚ���Ƭ�������Ҷˣ������ʱ�ĵ���I1�����Դ��ѹU=I1Rx��������Ƭ��������ˣ������ʱ�ĵ���I2�����Դ��ѹU=I2Rx+I2R������ʽ��![]() ��
��
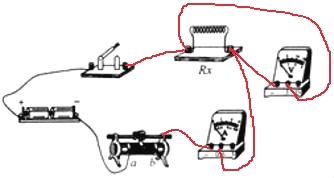
��������
�����������1�������ӵ�·ʱ����Ӧ�ô��ڶϿ�״̬����2����ͼ�ҿ�֪��ͨ��С���ݵĵ���I=0.3A��С�������˵ĵ�ѹU=2.4V����ʱС���ݵ�˿�ĵ���ֵR=![]() =
=![]() =8������ͼ�ҿ�֪����ѹ��������Ϊ0��3V��������������Ϊ0��0.6A��������������������Լ������������������ӣ���ѹ�������ڱ���������ˣ�������������һ��һ�µ�ԭ����ߣ�����ͼ��ʾ����3�������ѹ��ͻȻ����֪�����������������ֵΪR����Դ��ѹ���ֲ��䣬������ʱ��ʵ���·ͼ������ͼ��ʾ��aʵ�鲽�裺������ʱ���Ƚ���Ƭ�������Ҷˣ������ʱ�ĵ���I1�����Դ��ѹU=I1Rx��������Ƭ��������ˣ������ʱ�ĵ���I2�����Դ��ѹU=I2Rx+I2R��b�����Դ��ѹ���ֲ��䣬����I1Rx=I2Rx+I2R����Rx�ı���ʽΪ��Rx=
=8������ͼ�ҿ�֪����ѹ��������Ϊ0��3V��������������Ϊ0��0.6A��������������������Լ������������������ӣ���ѹ�������ڱ���������ˣ�������������һ��һ�µ�ԭ����ߣ�����ͼ��ʾ����3�������ѹ��ͻȻ����֪�����������������ֵΪR����Դ��ѹ���ֲ��䣬������ʱ��ʵ���·ͼ������ͼ��ʾ��aʵ�鲽�裺������ʱ���Ƚ���Ƭ�������Ҷˣ������ʱ�ĵ���I1�����Դ��ѹU=I1Rx��������Ƭ��������ˣ������ʱ�ĵ���I2�����Դ��ѹU=I2Rx+I2R��b�����Դ��ѹ���ֲ��䣬����I1Rx=I2Rx+I2R����Rx�ı���ʽΪ��Rx=![]() ��
��

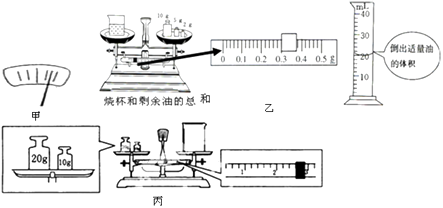
����Ŀ��ʵ��̽��������ʳ�����ܶȡ���ʵ�鷽�������������ǵķ��������ش���������⣺
��1��������ƽʱ������ָ��ƫ��ֶ��̵��Ҳ࣬��ͼ����ʾ����ʱӦ�������ϵ�ƽ����ĸ�����ڣ�ѡ����ߡ����ұߡ�����
��2��С���ķ����� �����ѵ���ƽ�����ƽ������ձ�������m1��
�����ձ��ڵ�������ʳ���ͣ��ٲ���ձ���ʳ���͵�������m2��
��Ȼ����ձ��е�ʳ����ȫ��������Ͳ�ڣ�������Ͳ��ʳ���͵����V
С����õ�ʳ���͵��ܶȵı���ʽ�� �� �������ϲ�������ĸд������ʽ��
��3��С���ķ����� �����ձ��ڵ���������ʳ���ͣ����ѵ���ƽ��õ���ƽ����ձ���ʳ���͵�������m3��
��Ȼ���ձ��ڵ�����ʳ���͵�����Ͳ�У��ٲ���ձ���ʣ��ʳ���͵�������m4 �� ������Ͳ��ʳ���͵����V2 �� С����õ�ʳ�����ܶȵı���ʽ�� �� ���������漰������ĸд������ʽ��
��4���Ƚ���λͬѧ��ʵ�鷽����ͬѧ��ʵ�����СһЩ�����ѡ����һ�ַ�������õ��ܶ�ֵ �� ��ѡ��ƫ��ƫС������
��5����ͼ����ʾ���ǰ���С����ʵ�鷽�����е�ij��ʵ���������ʵ������ݼ��������������У�
�ձ���ʳ���͵���������g�� | �ձ���ʣ���͵���������g�� | ����ʳ���͵����� | ����ʳ���͵������cm3�� | ʳ���͵��ܶȣ�g/cm3�� |
34.1 |
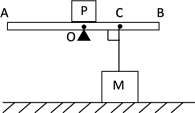
��6����ͼ����ʾ��һ�����ĵ�ͬѧ����������������̣�������������̣������������Ϊg��