��Ŀ����
С������ƽ����Ͳ��ˮ����һ��������ܶȣ�

��1��Ϊ�˲����������������С�����Ȱ���ƽ����ˮƽ�����ϣ���ͼ����ʾ���������IJ�����
a��______��
b����______��ѡ����/�ң�����ƽ����ĸ��ʹ��ƽƽ�⣻
c�������̷��Ͻ����飬�����̼Ӽ����벢�ƶ�����ʹ��ƽ����ƽ�⣬��ͼ����ʾ�������������m=______g��
��2��Ϊ�˲���������������С��������������������
a������Ͳ�е���һЩˮ�������Ͳ��ˮ�����V1����ͼ����ʾ��
b���ѽ�����ֱ��Ͷ��װ��ˮ����Ͳ�У�����ˮ���������Ŀ̶�V2����ͼ����ʾ����������ʵ������У�ָ�����ڵ���������֮��������������______����______��
��3����������������ñ���������õ����������ű�ʾ�������ܶȣ���=______��
�⣺��1����ƽ����ˮƽ̨�ϣ������Ƶ������˵���̶ȣ����ں�����ƽ����ĸ��ʹָ��ָ���ֶ��̵�����λ�ã����ָ��ƫ��̶ȱ�ߵ���࣬��ɽ�����ƽ��˵�ƽ����ĸ���ҵ������߽���ƽ�Ҷ˵�ƽ����ĸ���ҵ�����ָ��ָ�ڷֶ��̵�����ʱ����ʾ��ƽƽ���ˣ�
�����������Ϊm=50g+10g+5g+1.4g=66.4g��
��2��b����ͼ������û�н�û��ˮ�У��������ֵ��ƫС��
������ֱ�ӷ�����Ͳ�У����װ���Ͳ���ƣ���ʵ�����ģ�
��3��������������V=V2-V1��
��������ܶȣ���=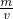 =
= ��
��
�ʴ�Ϊ����1��a�����������Ƶ���������̶ȴ���b���ң�c��66.4��
��2����ˮû�ܽ���������ȫ��û���ڽ�����Ҫ��ϸ�߰�ס������������Ͳ����3�� ��
��
��������1�����Ȱ���ƽ����ˮƽ̨�ϣ������Ƶ������˵���̶ȣ�����ƽ����ĸʹָ��ָ���ֶ��̵�����λ�ã�����ƽ��ʱ����ָ��ƫ��ķ�������ڣ�
��2���������������������������������������Կ̶�֮�ͣ���ȡ�������Կ̶�ʱ�������Ϊ��
��3������Ͳ��������������ʱ����Ͳ��Ҫ����������ˮ������ˮ�����V1���ѽ�������ϸ��˩�ã���û��ˮ�У�����ˮ�ͽ�����������V2�����������������
֪�������������������������ܶȹ�ʽ�����������ܶȣ�
��������1�����⿼�������ƽ��ʹ�ò��裬����ʹ�ù���������Ӧ���������յ���Ҫ���ݣ�
��2����Ͳ�е���������ˮ���������������壺ˮҪ�ܽ�û���壻�����û��ˮ�У�ˮ���������������ܳ�����Ͳ�����̣�
��3��������֪��ĸȥ������������
�����������Ϊm=50g+10g+5g+1.4g=66.4g��
��2��b����ͼ������û�н�û��ˮ�У��������ֵ��ƫС��
������ֱ�ӷ�����Ͳ�У����װ���Ͳ���ƣ���ʵ�����ģ�
��3��������������V=V2-V1��
��������ܶȣ���=
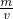 =
= ��
���ʴ�Ϊ����1��a�����������Ƶ���������̶ȴ���b���ң�c��66.4��
��2����ˮû�ܽ���������ȫ��û���ڽ�����Ҫ��ϸ�߰�ס������������Ͳ����3��
 ��
����������1�����Ȱ���ƽ����ˮƽ̨�ϣ������Ƶ������˵���̶ȣ�����ƽ����ĸʹָ��ָ���ֶ��̵�����λ�ã�����ƽ��ʱ����ָ��ƫ��ķ�������ڣ�
��2���������������������������������������Կ̶�֮�ͣ���ȡ�������Կ̶�ʱ�������Ϊ��
��3������Ͳ��������������ʱ����Ͳ��Ҫ����������ˮ������ˮ�����V1���ѽ�������ϸ��˩�ã���û��ˮ�У�����ˮ�ͽ�����������V2�����������������
֪�������������������������ܶȹ�ʽ�����������ܶȣ�
��������1�����⿼�������ƽ��ʹ�ò��裬����ʹ�ù���������Ӧ���������յ���Ҫ���ݣ�
��2����Ͳ�е���������ˮ���������������壺ˮҪ�ܽ�û���壻�����û��ˮ�У�ˮ���������������ܳ�����Ͳ�����̣�
��3��������֪��ĸȥ������������

��ϰ��ϵ�д�
 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
�����Ŀ


