��Ŀ����
�ع����к��д����������ж����к������ʡ�һЩ�������˶�����м���ˮ�����ӡ��ѳ�������ð��ɫ�������г������ۣ���ƭ���˺�����
С�����ò��ܶȵķ���������ɫ���ͺ͵ع��͡����ȣ���ͨ������������ɫ���͵��ܶ���0.91g/cm3 - 0.93g/cm3֮�䣬�ع��͵��ܶ���0.94g/cm3 - 0.95g/cm3֮�䡣Ȼ������������²������ʵ�����
A.��������Ʒ�͵�����Ͳ�к���ձ���ʣ����Ʒ�͵�������m��
B.����ƽ����ˮƽ̨���ϵ�ƽ��
C.ȡ������Ʒ�͵����ձ�������ƽ����ձ�����Ʒ�͵�������M��
D.������Ͳ����Ʒ�͵����V��
E.���ݲ�������ܶȣ�������Ʒ�͵�Ʒ�ʣ�
F.����ʵ�����ݣ��������Ʒ�͵��ܶȣ�
��1����3�֣��뽫����ʵ�鲽����ȷ���� ������ĸ��ţ� 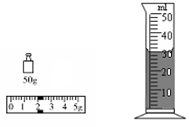
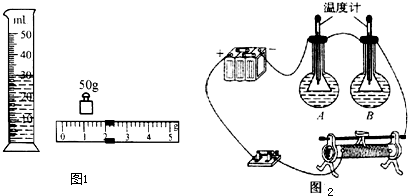
��2����2�֣���ͼʾ��֪��M= �ˣ���Ʒ�͵����V = ml��
��3����3�֣���m=23.8�ˣ�����Ʒ�͵��ܶ� g/cm3��
g/cm3��
��4����2�֣�С��ͨ���ȶԲ��������ܷ�϶���Ʒ���ǵع��ͣ�Ϊʲô��
Сǿ��Ϊ��������Ҳ�����ʵ�һ�����ԣ��Ƚϲ�ͬ���ʵı����ݣ�ͬ�����Լ������ǡ����ǣ�����ȡ������ȵ�ɫ���ͺ���Ʒ�ͣ��ֱ�װ��A��B������ƿ�ڣ���������ֵ��ͬ�ĵ���˿�ֱ����������ƿ�ڣ�����������·����ͼ��ʾ��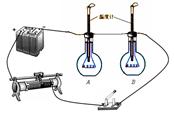
��5����3�֣�ʵ���У�Сǿ��������ֵ��ͬ�ĵ���˿���������·����Ŀ���� ��ȡ������ȵ�ɫ���ͺ���Ʒ�ͣ���Ŀ���� ��ͨ���۲� �����ܱȽϳ����DZ����ݵĴ�С�������˼������Ʒ�͵�Ʒ�ʡ�
��1��B��C��A��D��F��E��ֻҪ�Ŵ�һ��˳���÷֣���2��M=52.0g��V=30.0ml��3�� 0.94g/cm3����4��������ѧ������������˵������С��ͨ���ȶԲ����������Զ϶���Ʒ���ǵع��͡���ΪС�����������Ʒ�͵��ܶ��ڵع����ܶȷ�Χ֮�ڡ���ѧ����������˼��֮��ͬ�Ϳɸ��֣���С��ͨ���ȶԲ����������ܶ϶���Ʒ���ǵع��͡���Ϊ����ɫ���͵��ܶȺ͵ع��͵��ܶ�����ʵ������������Բ��ܶ϶���Ʒ���ǵع��͡���ѧ����������˼��֮��ͬ�Ϳɸ��֣���5������ȵ�ʱ���ڣ���������˿�ų���������ȡ���ѧ����������˼��֮��ͬ�Ϳɵ÷֣����Ʊ�������ͬ�����ȶԵȣ�ѧ����������˼��֮��ͬ�Ϳɵ÷֣� ��֧�¶ȼƵ�ʾ���仯��С��ѧ����������˼��֮��ͬ�Ϳɵ÷֣�
0.94g/cm3����4��������ѧ������������˵������С��ͨ���ȶԲ����������Զ϶���Ʒ���ǵع��͡���ΪС�����������Ʒ�͵��ܶ��ڵع����ܶȷ�Χ֮�ڡ���ѧ����������˼��֮��ͬ�Ϳɸ��֣���С��ͨ���ȶԲ����������ܶ϶���Ʒ���ǵع��͡���Ϊ����ɫ���͵��ܶȺ͵ع��͵��ܶ�����ʵ������������Բ��ܶ϶���Ʒ���ǵع��͡���ѧ����������˼��֮��ͬ�Ϳɸ��֣���5������ȵ�ʱ���ڣ���������˿�ų���������ȡ���ѧ����������˼��֮��ͬ�Ϳɵ÷֣����Ʊ�������ͬ�����ȶԵȣ�ѧ����������˼��֮��ͬ�Ϳɵ÷֣� ��֧�¶ȼƵ�ʾ���仯��С��ѧ����������˼��֮��ͬ�Ϳɵ÷֣�
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�