��Ŀ����
18����ͼ��ʾ����ijѧϰС��ͬѧ��Ƶ��о������������ǿ������ʵ���·ͼ��
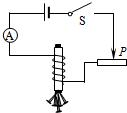
��1��Ҫ�ı�������Ȧ�еĵ�����С����ͨ��
��2���±��Ǹ���ͬѧ����ʵ��ļ�¼��
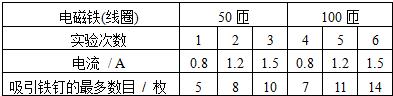
�ٱȽ�ʵ���е�1��2��3����4��5��6�����ɵó��Ľ����ǣ������������һ��ʱ��ͨ���������Ȧ�еĵ���
�ڱȽ�ʵ���е�1��4����2��5��3��6�����ɵó��Ľ����ǣ��������Ȧ�еĵ���һ��ʱ����Ȧ����
��3������ͬѧ�ǽ�������ʱ����һ���һ��ͬѧ���һ�����⣺������Ȧ�еĵ���������һ��ʱ��������Ĵ���ǿ����ỹ����Ȧ�ڵ���о��С�йأ���
����Դ˲����ǣ�
�����д�С��ͬ��������о�����ñ����·˵������֤����ķ�����

��1��Ҫ�ı�������Ȧ�еĵ�����С����ͨ��
�����������Ļ�ƬP���ƶ�
��ʵ�֣�Ҫ�жϵ�����Ĵ���ǿ�����ɹ۲������������ͷ�����Ŀ
��ȷ������2���±��Ǹ���ͬѧ����ʵ��ļ�¼��

�ٱȽ�ʵ���е�1��2��3����4��5��6�����ɵó��Ľ����ǣ������������һ��ʱ��ͨ���������Ȧ�еĵ���
Խ������Ĵ���Խǿ��
���ڱȽ�ʵ���е�1��4����2��5��3��6�����ɵó��Ľ����ǣ��������Ȧ�еĵ���һ��ʱ����Ȧ����
Խ�࣬������Ĵ���Խǿ��
����3������ͬѧ�ǽ�������ʱ����һ���һ��ͬѧ���һ�����⣺������Ȧ�еĵ���������һ��ʱ��������Ĵ���ǿ����ỹ����Ȧ�ڵ���о��С�йأ���
����Դ˲����ǣ�
������Ĵ���ǿ������Ȧ�е���о��С�����й�
�������д�С��ͬ��������о�����ñ����·˵������֤����ķ�����
��������ͬ����о������ͬ��������Ȧ�������Ǵ��������ͬһ��·��ͨ�磬�Ƚ�����������ͷ�����Ŀ�Ķ��٣�
���������ɵ�·ͼ��֪��������뻬�����������������ԣ���ƬP���ƶ������Ըı��·�еĵ������о�������Ĵ���ǿ�������ʵ�����õ��˿��Ʊ�������ת���������Ե�ǿ����ֱ�ӿ��������ģ����ô���ǿ����ͬ�������Ĵ�ͷ�����Ŀ��ͬ����ʶ�����ǿ���������һ��ת���������Ե�ǿ���ɵ�����С����Ȧ����������������������Ҫ�ÿ��Ʊ����������о���
����⣺��1���ɵ�·ͼ��֪��������뻬�����������������ԣ���ƬP���ƶ����ı们�������������·����ֵ���������Ըı��·�еĵ��������Ե�ǿ����ֱ�ӿ��������ģ����ô���ǿ����ͬ�������Ĵ�ͷ�����Ŀ��ͬ����ʶ�����ǿ���������һ��ת������
��2�����ɱ�������1��2��3��֪����Ȧ��������ͬ���������Ĵ�ͷ�����Ŀ��ͬ��������Խ�������Ĵ�ͷ��Խ�࣬��ͷ��Խ�࣬����Խǿ���ɴ˵ó����ۣ������������һ��ʱ��ͨ���������Ȧ�еĵ���Խ������Ĵ���Խǿ��
�ڱȽ�ʵ���е�1��4��֪��ͨ������Ȧ�ĵ�����ͬ������Ȧ������ͬ1��50�ѣ�2��100�ѣ���2�Ĵ��Ա�1��ǿ���ɴ˵ó����ۣ��������Ȧ�еĵ���һ��ʱ����Ȧ����Խ�࣬������Ĵ���Խǿ��
��3���ٲ�����Χ����������еģ���˲���Ϊ��������Ĵ���ǿ������Ȧ�е���о��С�����й�
�����ڴ��Ե�ǿ���Ѿ�֪���������С����Ȧ�����йأ����̽������о�Ĵ�С�Ƿ��й�ʱ��Ҫ���Ƶ�����С����Ȧ��������ͬ������о�Ĵ�С��ͬ���ʲ���Ϊ����������С��ͬ����о������ͬ��������Ȧ�������Ǵ��������ͬһ��·��ͨ�磬�Ƚ�����������ͷ�����Ŀ�Ķ��٣�
�ʴ�Ϊ����1���ƶ������������Ļ�Ƭ��������ͷ����Ŀ��
��2����Խ������Ĵ���Խǿ����Խ�࣬������Ĵ���Խǿ��
��3���ٵ�����Ĵ���ǿ������Ȧ�е���о��С�����йأ�����������ͬ����о������ͬ��������Ȧ�������Ǵ��������ͬһ��·��ͨ�磬�Ƚ�����������ͷ�����Ŀ�Ķ��٣�
��2�����ɱ�������1��2��3��֪����Ȧ��������ͬ���������Ĵ�ͷ�����Ŀ��ͬ��������Խ�������Ĵ�ͷ��Խ�࣬��ͷ��Խ�࣬����Խǿ���ɴ˵ó����ۣ������������һ��ʱ��ͨ���������Ȧ�еĵ���Խ������Ĵ���Խǿ��
�ڱȽ�ʵ���е�1��4��֪��ͨ������Ȧ�ĵ�����ͬ������Ȧ������ͬ1��50�ѣ�2��100�ѣ���2�Ĵ��Ա�1��ǿ���ɴ˵ó����ۣ��������Ȧ�еĵ���һ��ʱ����Ȧ����Խ�࣬������Ĵ���Խǿ��
��3���ٲ�����Χ����������еģ���˲���Ϊ��������Ĵ���ǿ������Ȧ�е���о��С�����й�
�����ڴ��Ե�ǿ���Ѿ�֪���������С����Ȧ�����йأ����̽������о�Ĵ�С�Ƿ��й�ʱ��Ҫ���Ƶ�����С����Ȧ��������ͬ������о�Ĵ�С��ͬ���ʲ���Ϊ����������С��ͬ����о������ͬ��������Ȧ�������Ǵ��������ͬһ��·��ͨ�磬�Ƚ�����������ͷ�����Ŀ�Ķ��٣�
�ʴ�Ϊ����1���ƶ������������Ļ�Ƭ��������ͷ����Ŀ��
��2����Խ������Ĵ���Խǿ����Խ�࣬������Ĵ���Խǿ��
��3���ٵ�����Ĵ���ǿ������Ȧ�е���о��С�����йأ�����������ͬ����о������ͬ��������Ȧ�������Ǵ��������ͬһ��·��ͨ�磬�Ƚ�����������ͷ�����Ŀ�Ķ��٣�
����������ƴ���ǿ������о��С��ʵ��ʱ��Ҫע�����ǰ���Ѿ�̽�������ľ������ص�����С����Ȧ������ͬ��

��ϰ��ϵ�д�
�����Ŀ
 ��2010?���ˣ���ͼ��ʾ����ijѧϰС��ͬѧ��Ƶ��о���Ӱ��ͨ�����߹ܴ���ǿ�������ء���ʵ���·ͼ��
��2010?���ˣ���ͼ��ʾ����ijѧϰС��ͬѧ��Ƶ��о���Ӱ��ͨ�����߹ܴ���ǿ�������ء���ʵ���·ͼ�� ��2007?������һģ����ͼ��ʾ����ijѧϰС��ͬѧ��Ƶ��о������������ǿ������ʵ���·ͼ��
��2007?������һģ����ͼ��ʾ����ijѧϰС��ͬѧ��Ƶ��о������������ǿ������ʵ���·ͼ��
 ��ͼ��ʾ����ijѧϰС��̽����������С����ʲô��ʵ����̣�
��ͼ��ʾ����ijѧϰС��̽����������С����ʲô��ʵ����̣�