��Ŀ����
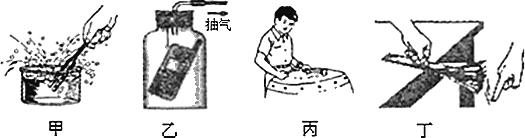
����Ŀ��ijͬѧϣ��ͨ���Ƚϵ�·�в�ͬλ�õĵ������Ķ������о�������·�ĵ������ɣ����ӵ�·ͼ��ͼ��ʾ���պϿ��غ���������ָ��ƫת�����ͼ��
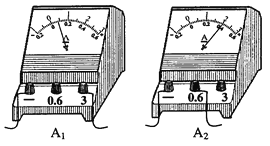
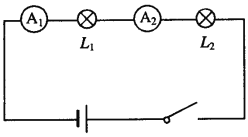
��1��������A2�Ķ����ǣ�____________________��
��2����ͬѧ���ֵ�����A1ָ��ƫת��A2С����������Ϊ��������·����ÿ����һ���õ����������������һЩ��������ָ��������жϴ����ԭ��______________________��
��3����ͬѧ�ҳ������ԭ������ܽ���˴�����·�е����Ĺ����ǣ�_________________________��
��4����һ��ͬѧ������ʵ��ʱ������ʵ���·���պϿ��أ�����ָ��Ѹ��ƫ���ұ�û�п̶ȵķ�Χ����������������ԭ����_______________����Ӧ�ò�ȡ�Ĵ�ʩ��______________________��
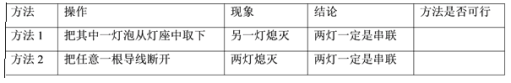
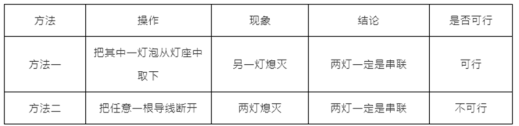
��5�����ӵ�·�������ݶ������������߽��ң�һʱ��ȷ����·�Ǵ������Dz������������ּ��жϷ����Ƿ���У������ڱ��пո���д�����С������С�________��
���𰸡�0.4A ��������ѡ���̲�ͬ ������·�����ĵ�������� ����������ѡС�� �Ͽ����أ����ӵ����������� 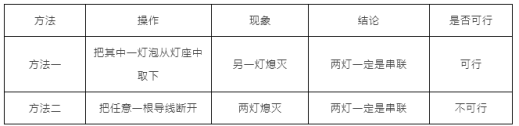
��������
��1������ͼ�е�����ѡ�õ�����ȷ���ֶ�ֵ������
��2����3��������·�����ĵ�������ȣ�û��ע��۲������������ʱ����ѡ��A1�����̽ϴ�ʱ������ֵ�����A1ָ��ƫת�Ƕȱ�A2С��
��4����������ʱ��������Ҫѡ����ʵ����̣�������ѡ�ù�С��ָ���ƫת���Ҷˣ���������
��5������1��������·ֻ��һ������·����������·�еĸ���·Ԫ���Ӱ�첻�ܶ����������ж��κ�һ�����ߣ����е��õ��������ܹ�����
����2��������·�ж�������·��������·Ԫ������Ӱ�죬�ܶ����������ж�һ��֧·������֧·�õ���������������������Ҫ�жϸ�·�����е��õ��������ܹ�����
��1��ͼ���У�������A2ѡ��С���̣��ֶ�ֵΪ0.02A�������ǣ�0.4A��
��2��������·�и����ĵ�����ȣ������ֵ�����A1ָ��ƫת�Ƕȱ�A2С��˵��û��ע��۲�����������̣����������������̲�һ����ɵģ�
��3��������·�����ĵ�������ȣ�
��4����һ��ͬѧ������ʵ��ʱ������ʵ���·���պϿ��أ�����ָ��Ѹ��ƫ���ұ�û�п̶ȵķ�Χ����������������ԭ���ǣ�����������ѡС�ˣ���Ӧ�ò�ȡ�Ĵ�ʩ�ǣ��Ͽ����أ����ӵ������Ĵ����̣�
��5������1��������һ�����ݴӵ�����ȡ�ߣ���������·����һ��Ϩ���ܹ�����˵�����ƻ���Ӱ�죬�����������Ǵ����ģ�����1���С�
����2���ڴ�����·�У��Ͽ�һ�����ߣ����е��õ��������ܹ������ڲ�����·�У��Ͽ���·���ߣ����е��õ���Ҳ�����ܹ���������Ͽ�һ�����ߣ����ƶ�Ϩ�������ݼ������Ǵ����ģ�Ҳ�����Dz��������ܸ��������ݶ�Ϩ���жϳ��������Ǵ����ģ��ʷ��������С�
�ʴ�Ϊ����1��0.4A����2����������ѡ���̲�ͬ����3��������·�����ĵ�������ȣ���4������������ѡС�ˣ��Ͽ����أ����ӵ������Ĵ����̣���5�����ϱ���