��Ŀ����
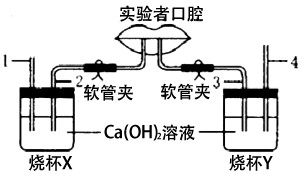
 ��Һͼ��ʾ�о����������������������ɷֲ����ʵ��װ�ã�ʵ�������Ҫ������ͨ��2�������壬ͨ��3�������壮
��Һͼ��ʾ�о����������������������ɷֲ����ʵ��װ�ã�ʵ�������Ҫ������ͨ��2�������壬ͨ��3�������壮 ��1��ʵ��װ�ô��ڴ���ָ��������������Ӧ���һ�㣺
2��4
2��4
����2����������ٶ�ʵ��װ������ȷ�ģ�
��3�����κ������ձ�X��Y����Һ����ɫ�ֱ���
�����ʯ��ˮû�б����
�����ʯ��ˮû�б����
�������ʯ��ʯ�����
�����ʯ��ʯ�����
����4������ú���ָʾ�����ȣ���ɫ��Χ��pH��3.1�ı�죬pH��4.4�ı�ƣ���NAHCO3��Һ����ͼ��Ca��OH��2��Һ�����κ�������ƿX��Y�ڵ���ɫ���ֱ���
��ɫ
��ɫ
����ɫ
��ɫ
����5���ӣ�3���пɵó����������������������ɷֵ�������
��������������к��н϶�Ķ�����̼����
��������������к��н϶�Ķ�����̼����
����6��������ͨ��2ʱ���伡������������״̬��
����
����
����ʱ���ڵ���ѹС��
��
����ѹ�������ε�ѪҺ�ı仯���ɾ���Ѫ��ɶ���Ѫ
�ɾ���Ѫ��ɶ���Ѫ
����7������ͨ��3ʱ���������ݻ��仯��
��С
��С
��������������
����
����8�������������������Ҫ��������
���
���
�ķֽ⣬�ͷ�����ά������ĸ�����������ڻ��������£�һ���е����ĵij�������ÿ����Ҫ����5858kJ����������ij�е����ĵij������ӣ�����ij�ּ������ܽ�ʳ��ֻ�ܿ���Һά�֣�����24h����������10%����������ҺԼ3416
3416
������������������һ��ʵ������⣬���ȴ�����۲��ʵ��װ�����֣�֪��������̼����ʹ�����ʯ��ˮ����ǵ����ԣ�
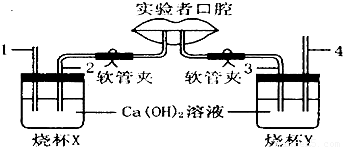
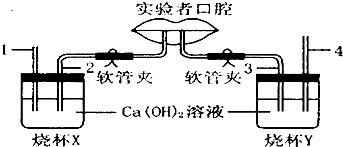
����⣺��1��ʵ�������Ҫ������ͨ��2�������壬ͨ��3�������壬���ձ�A��2�����ܲ���ʯ��ˮ�У��������������2������Ӧ���һ�㣬���ܲ���ʯ��ˮ�У��ձ�B��4�����ܵ�������������������4�����ܲ������ʯ��ˮ�У������������������4������Ӧ���һ�㣬���ܲ���ʯ��ˮ�У�
��3��X�ձ��ǽ����ڣ�Ϊ������������壬Y�ձ�Ϊ�����ڣ�Ϊ������������壬�˺���ʱ�������е�����ת��Ϊ������̼�����Ժ��������ж�����̼�ĺ����ȿ����еĶ�����̼�����ߣ�X�ձ��е�ʯ��ˮͨ�����ǿ���������ձ�X�ڵ������dz����ʯ��ˮû�б���ǣ�Y�ձ��е�ʯ��ˮͨ�����Ǻ��������壬���Y�ձ��еij����ʯ��ʯ����ǣ�
��4������ú���ָʾ�����ȣ���ɫ��Χ��pH��3.1�ı�죬pH��4.4�ı�ƣ���NAHCO3��Һ����ͼ��Ca��OH��2��Һ�����κ�������ƿX������ʾ���ԣ���˱��ɫ��Y�������˺���������к��ж�����̼����ʹ��Һ��ʾ���ԣ�������Һ����ɫΪ��ɫ��
��5������̽�������⣬��ʵ��ó���ʵ���������������������к��н϶�Ķ�����̼���壮
��6��������ͨ��2ʱ������������ʱ״̬����ʱ���⼡�������������߹����������ƶ������������½���������������ǻ�ݻ�����������š�������ѹ�����ڷ�����ѹ������������Σ������ε�ѪҺ�ı仯���ɾ���Ѫ��ɶ���Ѫ��
��7������ͨ��3ʱ��������ʱ���ں���״̬������ʱ�����⼡���������ţ����߹��½�������������������ǻ�ݻ���С���ν赯�Ի��������·�����ѹ��������������ų��Σ�
��8�������������������Ҫ���������л���ķֽ⣬�ͷ�����ά������ĸ������������֪���ڱ�״���£�ÿ��������[C6H12O6]�ֽ�ʱ����������Ϊ17.15ǧ����һ��ҹ������������10%��������ҺymL����5858��17.15=10%y�����y��3416mL
�ʴ�Ϊ��
��1��2��4��
��3�������ʯ��ˮû�б���ǣ������ʯ��ʯ����ǣ�
��4����ɫ ��ɫ
��5����������������к��н϶�Ķ�����̼����
��6������ С�� �ɾ���Ѫ��ɶ���Ѫ
��7����С ����
��8����� 3416
��3��X�ձ��ǽ����ڣ�Ϊ������������壬Y�ձ�Ϊ�����ڣ�Ϊ������������壬�˺���ʱ�������е�����ת��Ϊ������̼�����Ժ��������ж�����̼�ĺ����ȿ����еĶ�����̼�����ߣ�X�ձ��е�ʯ��ˮͨ�����ǿ���������ձ�X�ڵ������dz����ʯ��ˮû�б���ǣ�Y�ձ��е�ʯ��ˮͨ�����Ǻ��������壬���Y�ձ��еij����ʯ��ʯ����ǣ�
��4������ú���ָʾ�����ȣ���ɫ��Χ��pH��3.1�ı�죬pH��4.4�ı�ƣ���NAHCO3��Һ����ͼ��Ca��OH��2��Һ�����κ�������ƿX������ʾ���ԣ���˱��ɫ��Y�������˺���������к��ж�����̼����ʹ��Һ��ʾ���ԣ�������Һ����ɫΪ��ɫ��
��5������̽�������⣬��ʵ��ó���ʵ���������������������к��н϶�Ķ�����̼���壮
��6��������ͨ��2ʱ������������ʱ״̬����ʱ���⼡�������������߹����������ƶ������������½���������������ǻ�ݻ�����������š�������ѹ�����ڷ�����ѹ������������Σ������ε�ѪҺ�ı仯���ɾ���Ѫ��ɶ���Ѫ��
��7������ͨ��3ʱ��������ʱ���ں���״̬������ʱ�����⼡���������ţ����߹��½�������������������ǻ�ݻ���С���ν赯�Ի��������·�����ѹ��������������ų��Σ�
��8�������������������Ҫ���������л���ķֽ⣬�ͷ�����ά������ĸ������������֪���ڱ�״���£�ÿ��������[C6H12O6]�ֽ�ʱ����������Ϊ17.15ǧ����һ��ҹ������������10%��������ҺymL����5858��17.15=10%y�����y��3416mL
�ʴ�Ϊ��
��1��2��4��
��3�������ʯ��ˮû�б���ǣ������ʯ��ʯ����ǣ�
��4����ɫ ��ɫ
��5����������������к��н϶�Ķ�����̼����
��6������ С�� �ɾ���Ѫ��ɶ���Ѫ
��7����С ����
��8����� 3416
�����������ۺ���ǿ����һ�����Ѷȣ���Ҫϸ�Ľ��

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
�����Ŀ
 ��Һͼ��ʾ�о����������������������ɷֲ����ʵ��װ�ã�ʵ�������Ҫ������ͨ��2�������壬ͨ��3�������壮
��Һͼ��ʾ�о����������������������ɷֲ����ʵ��װ�ã�ʵ�������Ҫ������ͨ��2�������壬ͨ��3�������壮