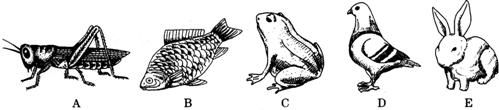
��Ŀ����
��ͼ��������ѧ�������ֶ����ش��й����⣺

��1��A��һ��ũҵ���棬��������ɷ�Ϊ
��2��B������ˮ�У����ĺ���������
��3��������Ⱥ���������������
��4��D��һ����ϡ����������
��5��E���

��1��A��һ��ũҵ���棬��������ɷ�Ϊ
ͷ
ͷ
����
��
����
��
�����֣���������������
�����
�������𱣻���֧�����ã����ܷ�ֹ����ˮ������
����ˮ������
����2��B������ˮ�У����ĺ���������
��
��
���������������Ĺ�������������ƽ��
��������ƽ��
������ǰ���������β��
�
��ǰ���Ķ����������ɲ���β���İڶ�
���ɲ���β���İڶ�
����3��������Ⱥ���������������
A
A
�����ں��¶������DE
DE
��������ĸ����4��D��һ����ϡ����������
������
������
�ͣ�����һ�ֶ��صĺ�����ʽ�������ڽṹ--����
����
�йأ���5��E��ë��
����
����
���ã����������ų�
�ų�
���ʳ�
�ʳ�
֮�֣�����ʳ������Ӧ��E��D��ȣ�����ֳ����������ص���̥������
̥������
�����������⿼���֪ʶ���Ƕ���ķ��࣮���ԴӶ��ﲻͬ���������������
����⣺��1���ȳ�������������������͵�������ȱ�����֧�����ڲ��ṹ��������Ч�ķ�ֹ����ˮ�ֵ����������ǽ�֫������Ӧ�ɺ�½���������Ҫԭ��
��2��B�����࣬������ˮ�У�����������������Ӿ���������������Ĺ����DZ�������ƽ�⣬����β������ǰ������ǰ���Ķ����������ɲ���β���İڶ���
��3��A�������������������BCDE�����ڶ��м��������ڼ����ABC�����²��㶨�����ڱ��¶��DE�����º㶨�����ں��¶������Խ�ߵ�ѧϰ����Խǿ�����鶯���Ƕ������ߵȵ�һ����Ⱥ�����ͼ�е�E���õ�ѧϰ������ǿ��
��4����������ǰԲ��⣬����⻬������ˮ�ε���״������������״���������������˶�ʱ���ܵ���������С���������������ɻ�������DZˮͧ�����γ����������ͣ� ������γ������ͱ�֤�������ܵ���С���������Ҹ�������кܶ����ң���Щ���������ͨ���Ҹ�����ʱ����������Σ��ڷ��ڽ������彻����ͬʱһ���ֿ�������������ʱ���棻����ʱ�������е������ֽ���Σ����ڷ��ڽ������彻�����������Ҹ�ÿ����һ�Σ��������ν���Σ��ڷ��ڽ����������彻���������ĺ�����ʽ����˫�غ�����˫�غ������������еĺ�����ʽ���������������彻����Ч�ʣ�
��5�����õ�ë�б������ã�����ʳ������Ӧ�����õ����ݷ�Ϊ�ųݺ;ʳݣ���Ȯ�ݣ��ųݳ������������벿�֣���״�����ӣ������ж�ʳ��ʳݳ������������࣬�п����ľ��棬����ĥ��ʳ����õ������ܺܳ����������ر��ä��������ʳ�������ܴ���������ֲ����ά��D�����࣬������E�Dz��鶯�����е���ֳ�����ص���̥������
�ʴ�Ϊ����1��ͷ���أ����������������ˮ��������2��������������ƽ�⣻β�������ɲ���β���İڶ� ��3��A��DE
��4������
��5�����£��ųݣ��ʳݣ�̥������
��2��B�����࣬������ˮ�У�����������������Ӿ���������������Ĺ����DZ�������ƽ�⣬����β������ǰ������ǰ���Ķ����������ɲ���β���İڶ���
��3��A�������������������BCDE�����ڶ��м��������ڼ����ABC�����²��㶨�����ڱ��¶��DE�����º㶨�����ں��¶������Խ�ߵ�ѧϰ����Խǿ�����鶯���Ƕ������ߵȵ�һ����Ⱥ�����ͼ�е�E���õ�ѧϰ������ǿ��
��4����������ǰԲ��⣬����⻬������ˮ�ε���״������������״���������������˶�ʱ���ܵ���������С���������������ɻ�������DZˮͧ�����γ����������ͣ� ������γ������ͱ�֤�������ܵ���С���������Ҹ�������кܶ����ң���Щ���������ͨ���Ҹ�����ʱ����������Σ��ڷ��ڽ������彻����ͬʱһ���ֿ�������������ʱ���棻����ʱ�������е������ֽ���Σ����ڷ��ڽ������彻�����������Ҹ�ÿ����һ�Σ��������ν���Σ��ڷ��ڽ����������彻���������ĺ�����ʽ����˫�غ�����˫�غ������������еĺ�����ʽ���������������彻����Ч�ʣ�
��5�����õ�ë�б������ã�����ʳ������Ӧ�����õ����ݷ�Ϊ�ųݺ;ʳݣ���Ȯ�ݣ��ųݳ������������벿�֣���״�����ӣ������ж�ʳ��ʳݳ������������࣬�п����ľ��棬����ĥ��ʳ����õ������ܺܳ����������ر��ä��������ʳ�������ܴ���������ֲ����ά��D�����࣬������E�Dz��鶯�����е���ֳ�����ص���̥������
�ʴ�Ϊ����1��ͷ���أ����������������ˮ��������2��������������ƽ�⣻β�������ɲ���β���İڶ� ��3��A��DE
��4������
��5�����£��ųݣ��ʳݣ�̥������
��������������Ŀ�Ĺؼ�����Ƕ������Ҫ��������ע�������⣮

��ϰ��ϵ�д�
�����Ŀ