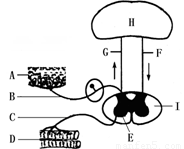
��Ŀ����
�������ϵͳ��ģʽͼ����ͼ���ش�
��1��ͼ��A�ṹ�ں��ио���ĩ�ң�������������ַ��䣬��д�����ַ���ķ���;������ͼ����ĸ��ʾ�� ��
��2������ֻ���ڿ����Լ���֫������£���֪����֫�ľ������������˿��ܵ����˲�λ�� ��A��F B��B C��C D��G
��3���ɣ�1������2����֪��������� �� ���ܡ�
��4��ͼ��I��Ҫ������ά��ɣ��ⲿ�ֵ������� ��
��5���Ͽ�ʱС������ʦ��Ҫ��̲���ijһҳ��������� �����Ҫ������Ҫ����H�IJ��롣
��1��A��B��E��C��D ��2��D ��3������ ������4������ ��5����Ҫ
��������
�����������1�����ڵĻ�����ʽ�Ƿ��䣬�����Ľṹ������Ϊ���仡�������������������������ࡢ������ЧӦ�����۲�ͼʾB�����������ڻ��������E�����ڴ�����ǰ�˻��Ƚ�С����ˡ����ַ��䡱��ķ���;����A��������B������E�������C������DЧӦ����
��2������ֻ���ڿ����Լ���֫������£���֪����֫�ľ�������������������֫�ܻ�����仡������������֧����֫�Ļ�����Dz��ܸ�֪��֫��λ�ã����ܵ����˲�λ��ͼG���д���������ά�����ܰ���֫�Ļ���ͨ��I������ʵ�����ά���д�����H����Ƥ�������о����࣬��˲��ܸ�֪��֫�ľ����������ˣ�ֻ���ڿ����Լ���֫������£���֪����֫�ľ��������
��3���ɣ�1����2����֪��������ܶ����Ĵ̼�������Ӧ�����ܽ�����Ӧ�������Ե�һ����������˼�����з��书�ܡ��������ܣ�
��4����������ϵͳ�����ಿ�֣�λ�������棬�϶��������裬���Է����ɶԵ����ֲ�����֫����ں����ࣻ����Ľṹ����λ�����벿�Ļ���ͼE��λ����Χ���İ���ͼI�������ڵ�����ά�ڼ���ĸ�����֮�䡢�Լ���������֮�䣬������ϵ���ã�ͼ��E��ɫ����IJ�λ����Ԫϸ���弯�еIJ�λ���ܽ����嶯�������µ��嶯��ͼ��I��ɫ��dz�IJ���������ά���еIJ�λ�а��ʣ�λ�ڼ����ڲ�����Χ���д������ܣ�����ͼ��IΪ���ʣ�
��5��������Ҫ�ǶԼ�����ӷ�����жϣ������븴�ӷ���ı��������Ƿ��д���Ƥ��IJ��룬û�д���Ƥ�����ģ��������ڴ���Ƥ�����µķ����Ǽ��䣬������������ڴ���Ƥ���ϵķ����Ǹ��ӷ��䣬�Ͽ�ʱС������ʦ��Ҫ��̲���ijһҳ�����������е��д���Ƥ�����������IJ����γɵĸ��ӷ��䣬�����Ҫ����Ƥ����������ɵķ�����
���㣺���⿼����Ƿ���ṹ�������仡����ɡ�������ɼ��书�ܡ��Լ���������ͣ���������Ҫ�������ͼʾ������ͼʾ�е���ĸ����ʾ�Ľṹ��

 �Ķ��쳵ϵ�д�
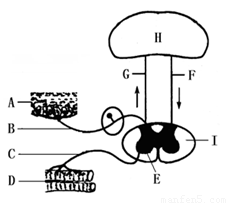
�Ķ��쳵ϵ�д�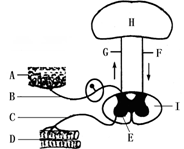
��1��ͼ��A�ṹ�ں��ио���ĩ�ң�������������ַ��䣬��д�����ַ���ķ���;������ͼ����ĸ��ʾ�� ��
��2������ֻ���ڿ����Լ���֫������£���֪����֫�ľ������������˿��ܵ����˲�λ�� ��
| A��F | B��B | C��C | D��G |
��4��ͼ��I��Ҫ������ά��ɣ��ⲿ�ֵ������� ��
��5���Ͽ�ʱС������ʦ��Ҫ��̲���ijһҳ��������� �����Ҫ������Ҫ����H�IJ��롣
 ��2011?����ģ�⣩�������ϵͳ��ģʽͼ�ش��������⣺
��2011?����ģ�⣩�������ϵͳ��ģʽͼ�ش��������⣺ ��2010?�е£��������ϵͳ��ģʽͼ����ͼ���ش�
��2010?�е£��������ϵͳ��ģʽͼ����ͼ���ش� ��2012?�ڶ���ģ�⣩�������ϵͳ��ģʽͼ�ش��������⣺
��2012?�ڶ���ģ�⣩�������ϵͳ��ģʽͼ�ش��������⣺