��Ŀ����
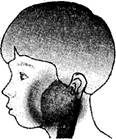
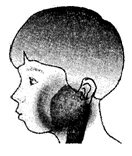
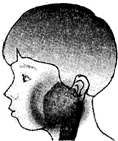
�����������ף��׳ơ��������������������Ƕ�ͯ���������г����ĺ�������Ⱦ��������2�·���������ʡ���������������Ⱥ�������Ⱦ������������������������������������֢״��Ҫ�Ƿ��ȡ��������ѣ������״�(����ͼ)��һ�����ߵ��������ף�Ӧ����������������ȫ���˺�ſ����л���ѧ�����Ⱦ���ˡ���Ȼ��߿���������ȥ��Ƥ�̣���������ڻ�������������ֹʹ���á���ش���������(5��)��
(1)�����������IJ�ԭ����Ҫ�������ײ�����������ҩƷ����û�����Ե���Ч���ӽṹ�Ϸ���������ϸ���������� ��
(2)�����ˮ���ֱ�ճĤʪ������Ч�������������֡��ӻ��;���Ͽ��������������� ��
(3)ijУij��ѧ�������˼���������Ͽ�����Ԥ����Ⱦ�����еĴ�ʩ�Ͽ��������� ��
(4)����ͬѧ��û�л��������������ף�����˵�Լ����ֹ��������磬���Բ����Ⱦ�����������ף�����Ϊ����ͬѧ��˵������? �����Խ���ԭ��
��
(1)û��ϸ���ṹ (2)������������ (3)�жϴ���;��
(4)���� �������������(����ֻ���ض��IJ�ԭ��������)
����

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�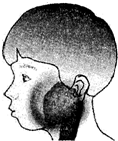 ��2012?���ݣ������������ף��׳ơ��������������������Ƕ�ͯ���������г����ĺ�������Ⱦ��������2�·���������ʡ���������������Ⱥ�������Ⱦ������������������������������������֢״��Ҫ�Ƿ��ȡ��������ѣ������״���ͼ����һ�����ߵ��������ף�Ӧ����������������ȫ���˺�ſ����л���ѧ�����Ⱦ���ˣ���Ȼ��߿���������ȥ��Ƥ�̣���������ڻ�������������ֹʹ���ã���ش��������⣺
��2012?���ݣ������������ף��׳ơ��������������������Ƕ�ͯ���������г����ĺ�������Ⱦ��������2�·���������ʡ���������������Ⱥ�������Ⱦ������������������������������������֢״��Ҫ�Ƿ��ȡ��������ѣ������״���ͼ����һ�����ߵ��������ף�Ӧ����������������ȫ���˺�ſ����л���ѧ�����Ⱦ���ˣ���Ȼ��߿���������ȥ��Ƥ�̣���������ڻ�������������ֹʹ���ã���ش��������⣺