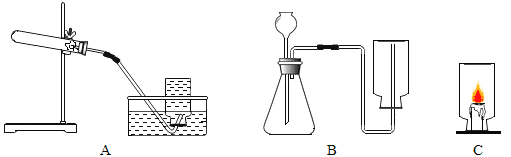
��Ŀ����
����Ŀ���о���ѧϰС���ͬѧ�������װ��̽������ɫֲ������������Ƿ���CO2�������������ش����⡣

���ϣ�����ɫֲ���ڱܹ�ĺڰ��������������ã�����Ϊ���л���+����![]() ˮ+����
ˮ+����
��2NaOH+CO2=Na2CO3+H2O
��1��װ����B���ֵ�������_____��
��2��C���ֲ��ò����ܱղ����ֵ�ԭ����_____��
��3��D��Ӧ�۲쵽��������_____��
��4���۹�����ʵ��װ�ã����A��B���ֵ�Ŀ���Ƿ�ֹ_____��
���𰸡���������е�CO2�Ƿ��� ʹ��ɫֲ��ֻ������������ �����ʯ��ˮ����� �����е�CO2����̽�����
��������
̽������ɫֲ������������Ƿ���CO2��������������У�
��1��������ж�����̼��ʵ����Ӱ�죬װ��A�����տ����ж�����̼������B�Ǽ�������еĶ�����̼�Ƿ�����
��2����ɫֲ���ڱܹ�ĺڰ���ֻ�����������ã�������������ã�C���ֲ��ò����ܱղ����ֵ�ԭ����ʹ��ɫֲ����������ã�
��3��Dװ���ó���ʯ��ˮ����ֲ�����ʱ����������̼�����D�г���ʯ��ˮ�����ǣ�
��4�� ��Ϊ�����ж�����̼��ʵ����Ӱ�죬���A��B���ֵ�Ŀ���Ƿ�ֹ�����е�CO2����̽�������

����Ŀ������ʵ������ܴﵽʵ��Ŀ���ǣ� ��
ѡ�� | ʵ��Ŀ�� | ʵ����� |
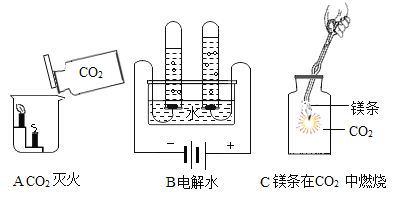
A | ����ľ̿�ۺ����� | ��ϡ���ᣬ�۲��������ݲ��� |
B | �������Ͷ�����̼ | ��ȼ�ŵ�ľ���ֱ�����ʢ����ļ���ƿ�� |
C | ��ȥһ����̼�е����� | �����建��ͨ�����ȵ�ͭ�� |
D | ��ȥFeSO4��Һ��������CuSO4 | ��������п�ۣ���ַ�Ӧ����� |
A.AB.BC.CD.D
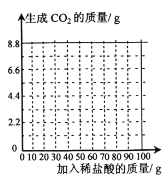
����Ŀ���������ƾ��û�����Ϊ̼��ơ�ʵ������һƿ���ֱ��ʵ�����������Ʒ��Ϊ�ⶨ����̼��Ƶ�����������ȡ16.2g��Ʒ���ձ��У���������ˮ���ٽ�100gϡ�������������ձ��в����Ͻ��裬�������������±�����ʾ�����������������ᷴӦʱ���������������ش����Ⲣ���㡣
����ϡ���������/g | 20.0 | 40.0 | 60.0 | 80.0 | 100 |
����CO2��������g | 0 | 2.2 | 4.4 | m | 5.5 |
��1���������Ʊ�������������е�____________�����˷�Ӧ��
��2������m����ֵΪ_____________��
��3���Լ�����Ʒ��̼��Ƶ���������___________�����ڴ����д��������̣���������ȷ��0.1%����
��4�����������л�������ϡ���������������CO2������֮��ı仯��ϵͼ___________��