��Ŀ����
��֪��Na2CO3���Ȳ��ֽ⣬2NaHCO3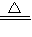 Na2CO3+CO2��+H2O��ij������Ʒ�л���������̼�����ƣ�Ϊ�˲ⶨ��Ʒ�Ĵ��ȣ���ѩͬѧ��������ʵ�飺ȷ��ȡ��Ʒ10.0g�����Թ��У����ȳ�ַ�Ӧ��������ų���������CO2����224mL����������������ܶ�Ϊ1.964g/L������ش��������⣺
Na2CO3+CO2��+H2O��ij������Ʒ�л���������̼�����ƣ�Ϊ�˲ⶨ��Ʒ�Ĵ��ȣ���ѩͬѧ��������ʵ�飺ȷ��ȡ��Ʒ10.0g�����Թ��У����ȳ�ַ�Ӧ��������ų���������CO2����224mL����������������ܶ�Ϊ1.964g/L������ش��������⣺��1������CO2������Ϊ______g����ȷ��0.01g����
��2��������Ʒ�е�Na2CO3�����������Ƕ��٣���Ҫ��д��������̣������ȷ��0.1%��
��3������ag�ô�����Ʒ��ּ�����������ų�����ȴ��Ƶ�ʣ�������������Ϊbg��b��a������ô�����Na2CO3�����������ı���ʽΪ______��Ҫ��д��������̣�����ܰ��ʾ��������̾��������ˣ���������ô���ռ䣬���ɳ������ⷶΧ��
���𰸡���������1����m=��v��������CO2��������
��2��������CO2������������Ϸ���ʽ������Ʒ�е�Na2CO3������������
��3�����ݷ�Ӧ���ٵ�����������ˮ�Ͷ�����̼���������ɷ�Ӧǰ�����������Լ����������Ӧ��̼�����Ƶ����������������������Na2CO3������������
����⣺��1������CO2������Ϊ��0.224L×1.964g/L��0.44g��
��2������Ʒ�е�Na2CO3����������Ϊx��
2NaHCO3 Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2��
168 44
10.0g��1-x�� 0.44g
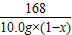 =
=
x=83.2%
��3���贿����Na2CO3����������Ϊy��
2NaHCO3 Na2CO3+H2O+CO2�� ���ٵ�����
Na2CO3+H2O+CO2�� ���ٵ�����
168 18 44 18+44=62
ag��1-y�� ag-bg
 =
=
y= %��
%��
�ʴ�Ϊ����1��0.44g��
��2��������Ʒ�е�Na2CO3������������83.2%��
��3���贿����Na2CO3����������Ϊy��
2NaHCO3 Na2CO3+H2O+CO2�� ���ٵ�����
Na2CO3+H2O+CO2�� ���ٵ�����
168 18 44 18+44=62
ag��1-y�� ag-bg
 =
=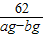
y= %
%
�𣺴�����Na2CO3������������ %��
%��
������������Ҫ�����йػ�ѧ����ʽ�ļ��㣬�ѶȽϴɽ�������غ㶨�ɽ��з���������ȽϷ�����Ҫϸ�ķ������
��2��������CO2������������Ϸ���ʽ������Ʒ�е�Na2CO3������������
��3�����ݷ�Ӧ���ٵ�����������ˮ�Ͷ�����̼���������ɷ�Ӧǰ�����������Լ����������Ӧ��̼�����Ƶ����������������������Na2CO3������������
����⣺��1������CO2������Ϊ��0.224L×1.964g/L��0.44g��
��2������Ʒ�е�Na2CO3����������Ϊx��
2NaHCO3
 Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2�� 168 44
10.0g��1-x�� 0.44g
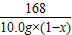 =
=
x=83.2%
��3���贿����Na2CO3����������Ϊy��
2NaHCO3
 Na2CO3+H2O+CO2�� ���ٵ�����
Na2CO3+H2O+CO2�� ���ٵ����� 168 18 44 18+44=62
ag��1-y�� ag-bg
 =
=
y=
 %��
%���ʴ�Ϊ����1��0.44g��
��2��������Ʒ�е�Na2CO3������������83.2%��
��3���贿����Na2CO3����������Ϊy��
2NaHCO3
 Na2CO3+H2O+CO2�� ���ٵ�����
Na2CO3+H2O+CO2�� ���ٵ����� 168 18 44 18+44=62
ag��1-y�� ag-bg
 =
=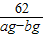
y=
 %
%�𣺴�����Na2CO3������������
 %��
%��������������Ҫ�����йػ�ѧ����ʽ�ļ��㣬�ѶȽϴɽ�������غ㶨�ɽ��з���������ȽϷ�����Ҫϸ�ķ������

��ϰ��ϵ�д�
�����Ŀ
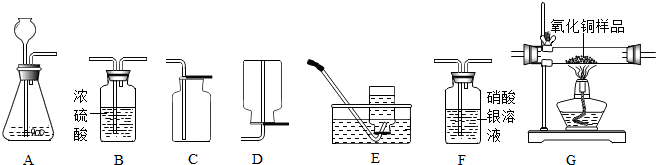
 A B C D E F G
A B C D E F G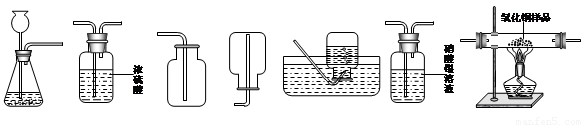 A
B
C
D
E
F G
A
B
C
D
E
F G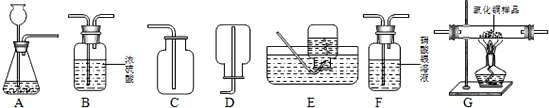
 Cu+H2O��
Cu+H2O��
 Cu+H2O��
Cu+H2O��