��Ŀ����
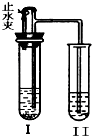 A��BΪ���л�ѧ�������ʣ����Ƿֱ���H��C��O��Cl��Ca��Mn�е�һ�ֻ���Ԫ����ɣ�����ͼװ�ã�ͼ������̨������������ȥ������ʵ�飮ʵ�鲽�����£�
A��BΪ���л�ѧ�������ʣ����Ƿֱ���H��C��O��Cl��Ca��Mn�е�һ�ֻ���Ԫ����ɣ�����ͼװ�ã�ͼ������̨������������ȥ������ʵ�飮ʵ�鲽�����£���1�����ڢ��м���һ��������A��Һ��B��������Ƥ����������ֹˮ�У����֢���������ð������A��B����Ϊ������д������Ҫ���2�����ʣ�
| A | |||
| B |
��2����һ��ʱ��ر�ֹˮ�У����֢���Һ������������Һ�ɻ��DZ���壮�����ԭҺ����
��2�����ݶ�����̼��ʹ����ʯ��ˮ����ǽ��н��
��2��������̼��ʹ����ʯ��ˮ����ǣ����Ԣ���ԭҺ���dz����ʯ��ˮ�����е��ܿ�������ð������Һ���ǣ���֪����������Ϊ������̼����Һ���ǵķ�Ӧ��CO2+Ca��OH��2=CaCO3��+H2O���ر�ֹˮ�У����в������ɵĶ�����̼ʹװ���ڵ���ѹ�����ˢ������������н����ɵ�̼�����ȫ��Ӧ���������Һ�ɻ��DZ���壬��Ӧ�Ļ�ѧ����ʽΪCaCO3+2HCl�TCaCl2+H2O+CO2����
�ʴ�Ϊ����1��
| A | ������ | �������� | ʯ��ʯ |
| B | ˮ | ����������Һ | ϡ���� |
��2�������ʯ��ˮ��CO2+Ca��OH��2=CaCO3��+H2O��CaCO3+2HCl=CaCl2+H2O+CO2����

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д� ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�A��BΪ���л�ѧ�������ʣ����Ƿֱ���H��C��O��Cl��Ca��Mn�е�һ�ֻ���Ԫ����ɡ�����ͼװ�ã�ͼ������̨������������ȥ������ʵ�顣ʵ�鲽�����£�
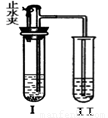
��1������I�м���һ��������A��Һ��B��������Ƥ����������ֹˮ�У����֢���������ð������A��B����Ϊ������д������Ҫ���2�����ʣ�
|
A |
|
|
|
|
B |
|
|
|
������ʾ����д���ʵĻ�ѧʽ�����ƻ��׳ơ�
��2����һ��ʱ��ر�ֹˮ�У����֢���Һ������������Һ�ɻ��DZ���塣�����ԭҺ���� ������ʵ������У����з�����Ӧ�Ļ�ѧ����ʽΪ ��
A��BΪ���л�ѧ�������ʣ����Ƿֱ���H��C��O��Cl�� Ca��Mn�е�һ�ֻ���Ԫ����ɡ�����ͼװ�ã�ͼ������̨������������ȥ������ʵ�顣
Ca��Mn�е�һ�ֻ���Ԫ����ɡ�����ͼװ�ã�ͼ������̨������������ȥ������ʵ�顣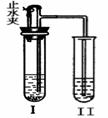 ʵ�鲽�����£�
ʵ�鲽�����£�
��1������I�м���һ��������A��Һ��B��������Ƥ��������
��ֹˮ�У����֢���������ð������A��B����Ϊ������д
������Ҫ���2�����ʣ�
| A | |||
| B |
������ʾ����д���ʵĻ�ѧʽ�����ƻ� �׳ơ�
�׳ơ�
��2����һ��ʱ��ر�ֹˮ�У����֢���Һ������������Һ�ɻ��DZ���塣�����ԭ
Һ����______������ʵ������У����з�����Ӧ�Ļ�ѧ����ʽ Ϊ_________________________________________________________________��
Ϊ_________________________________________________________________��
 ��2013?���ݣ���֪A-H��Ϊ���л�ѧ���������ʣ�����A��C�����Ԫ����ͬ�����壬��C�ܲ�������ЧӦ��BΪ����ɫ���dz��������Ҫ�ɷ֣�F����ɫ������H����ɫ����������ͼʾ��ת����ϵ��ͼ�з�Ӧ����������ȥ������ش�
��2013?���ݣ���֪A-H��Ϊ���л�ѧ���������ʣ�����A��C�����Ԫ����ͬ�����壬��C�ܲ�������ЧӦ��BΪ����ɫ���dz��������Ҫ�ɷ֣�F����ɫ������H����ɫ����������ͼʾ��ת����ϵ��ͼ�з�Ӧ����������ȥ������ش�