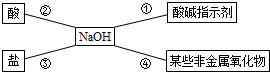
��Ŀ����
��2012?ͭ�ʵ�������ѧ�����������ߣ�������������������ϢϢ��أ��������³��������ʣ�
��CO2����CH3COOH����NaOH����NaCl����Na2CO3����C2H5OH����SO2
��ѡ��������ʵ������գ�
��1���׳ƴ������
��2��ҽ������������������ˮ����
��3��������Ҫ������Ⱦ��֮һ����
��4����ɫֲ����й��������Ҫ����
��5��ʳ���к��е���
��6������ę́�к��е���
��CO2����CH3COOH����NaOH����NaCl����Na2CO3����C2H5OH����SO2
��ѡ��������ʵ������գ�
��1���׳ƴ������
��
��
����2��ҽ������������������ˮ����
��
��
����3��������Ҫ������Ⱦ��֮һ����
��
��
����4����ɫֲ����й��������Ҫ����
��
��
����5��ʳ���к��е���
��
��
����6������ę́�к��е���
��
��
��������Na2CO3�����Ǵ��NaCl��ҽ������������������ˮ��SO2���ڿ�����Ⱦ���ɫֲ����й��������Ҫ���Ƕ�����̼��ˮ��CH3COOH�Ǵ������Ҫ�ɷ֣��Ƶ���Ҫ�ɷ���C2H5OH��
����⣺��1��Na2CO3�����Ǵ������ݣ�
��2��NaCl��ҽ������������������ˮ������ܣ�
��3��SO2���ڿ�����Ⱦ�����ߣ�
��4����ɫֲ����й��������Ҫ���Ƕ�����̼��ˮ������٣�
��5��CH3COOH�Ǵ������Ҫ�ɷ֣�����ڣ�
��6���Ƶ���Ҫ�ɷ���C2H5OH�������
��2��NaCl��ҽ������������������ˮ������ܣ�
��3��SO2���ڿ�����Ⱦ�����ߣ�
��4����ɫֲ����й��������Ҫ���Ƕ�����̼��ˮ������٣�
��5��CH3COOH�Ǵ������Ҫ�ɷ֣�����ڣ�
��6���Ƶ���Ҫ�ɷ���C2H5OH�������
���������⿼����л�ѧ�г����ļ������ʵ���;����Ŀ�Ƚϼ���ƽʱע�⽫��ѧ֪ʶ������������ϵ��һ����ô�����������ģ�

��ϰ��ϵ�д�
�����Ŀ