��Ŀ����
ͨ��һ��Ļ�ѧѧϰ������֪���˳��������ʵ������ȡ������������ʣ�������ѧ֪ʶ�ش��������⣺
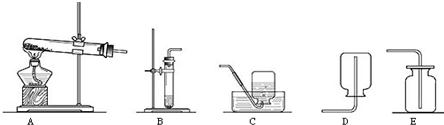
����ͼ��ʵ������ȡ����ʱ���õ�װ�ã�
��д��װ��A������Ӧ���������������� ��
��ʵ�����ù���������Һ��������̻����ȡ�����Ļ�ѧ����ʽΪ ����ѡ�õķ���װ���� (�����)����Eװ���ռ��������ж������Ѽ����������� ��
��ʵ���ҳ��ü����Ȼ�狀��������ƵĹ�����������ȡ������Ϊ����ȡ���ռ�������İ�������ѡ�õ�װ������� (�����)��
��ʹ������ʱ��Ҫ�ر�ע�ⰲȫ��ͼ21-2�Ǽ����������ȵ�ʵ�飬�ݴ����ܵó���������������� �� ��
����ͼ21��3��������������ȼ��ʵ��ʱ��ʢ���ȼ�ճ�Ӧ��ƿ�����»������룬ԭ���� ��ʵ���й۲쵽 ���棬ƿ��װˮ�������������ж��Ķ������������ˮ����һ����ҺЧ�����ã����ָ���Һ���õĻ�ѧ����ʽΪ ��

ͼ21����2 ͼ21����3

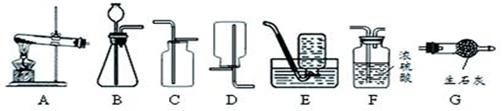
����ͼ��ʵ������ȡ����ʱ���õ�װ�ã�
��д��װ��A������Ӧ���������������� ��
��ʵ�����ù���������Һ��������̻����ȡ�����Ļ�ѧ����ʽΪ ����ѡ�õķ���װ���� (�����)����Eװ���ռ��������ж������Ѽ����������� ��
��ʵ���ҳ��ü����Ȼ�狀��������ƵĹ�����������ȡ������Ϊ����ȡ���ռ�������İ�������ѡ�õ�װ������� (�����)��
��ʹ������ʱ��Ҫ�ر�ע�ⰲȫ��ͼ21-2�Ǽ����������ȵ�ʵ�飬�ݴ����ܵó���������������� �� ��
����ͼ21��3��������������ȼ��ʵ��ʱ��ʢ���ȼ�ճ�Ӧ��ƿ�����»������룬ԭ���� ��ʵ���й۲쵽 ���棬ƿ��װˮ�������������ж��Ķ������������ˮ����һ����ҺЧ�����ã����ָ���Һ���õĻ�ѧ����ʽΪ ��

ͼ21����2 ͼ21����3
��ÿ������ʽ2�֣�����ÿ��1�֣�
MnO2
��1�����Թ� �� 2H2O2===2H2O + O2�� �� B ��ƿ�������ݲ���
��A D G
(2)�ܶȱȿ���С����ȼ��
��3����ֹ�������ݡ�����ɫ SO2 + 2NaOH ="=" Na2SO3 + H2O
MnO2
��1�����Թ� �� 2H2O2===2H2O + O2�� �� B ��ƿ�������ݲ���
��A D G
(2)�ܶȱȿ���С����ȼ��
��3����ֹ�������ݡ�����ɫ SO2 + 2NaOH ="=" Na2SO3 + H2O
���⿼�����ʵ������ȡ������й�֪ʶ�����п��ؿ��IJ��֣�������Ҫ�����ȡ������������̼���������й�֪ʶ��ͬʱҪ���շ���װ�ú��ռ�װ�õ�ѡ��ԭ�������ȹ�����ȡ���壬��ѡ����ø��������ȡ�����ķ���װ��һ������Һ��ϣ�����Ҫ���Ⱦ�ѡ����ȡ������̼�ķ���װ�ã��ռ�װ�ÿ��������ʣ��ܶȱȿ������������ſ��������ܶȱȿ���С���������ſ�������������ˮ��������ˮ��������ˮ�������Լ����Ȼ�狀��������ƵĹ�����������ȡ����������װ��ѡ��A,�ռ�װ��ѡ��D,��Ϊ�����Լ��ԣ�������Ũ��������������ʯ�Ҹ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 CaCl2 + 2NH3��+ 2H2O
CaCl2 + 2NH3��+ 2H2O