��Ŀ����
����A��B��C��D��E��F���dz��л�ѧ�еij������ʡ�����������ͼ��ʾ��ת����ϵ���ش��������⣺
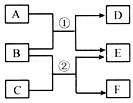
(1)��D��һ�ֺ�ɫ��������D��C����Һ���ܷ�����ѧ��Ӧ����A�Ļ�ѧʽΪ____����Ӧ�ڵĻ�ѧ����ʽΪ______________��
(2)��E������ˮ����C��D��Һ�ܷ�����ѧ��Ӧ��д����Ӧ�١��ڵĻ�ѧ����ʽ��
��___________________________����____________________________��
(3)��A��B��C�����������ͬ��D��F��������ˮ����D����Է�������С��F����C�Ļ�ѧʽΪ____����Ӧ�ٵĻ�ѧ����ʽΪ_____________________��
(2)��E������ˮ����C��D��Һ�ܷ�����ѧ��Ӧ��д����Ӧ�١��ڵĻ�ѧ����ʽ��
��___________________________����____________________________��
(3)��A��B��C�����������ͬ��D��F��������ˮ����D����Է�������С��F����C�Ļ�ѧʽΪ____����Ӧ�ٵĻ�ѧ����ʽΪ_____________________��
(1)CuSO4��Fe+2H2SO4==FeSO4+H2��
(2)��Na2CO3+Ca(OH)2==2NaOH+CaCO3������CO2+Ca(OH)2==CaCO3��+H2O
(3)Fe2O3��HCl+NaOH==NaCl+H2O (��CO2��HCl+NaOH==NaCl+H2O) �������������𰸾��÷֡�
(2)��Na2CO3+Ca(OH)2==2NaOH+CaCO3������CO2+Ca(OH)2==CaCO3��+H2O
(3)Fe2O3��HCl+NaOH==NaCl+H2O (��CO2��HCl+NaOH==NaCl+H2O) �������������𰸾��÷֡�

��ϰ��ϵ�д�
�����Ŀ
 23��ijͬѧѧϰ�����з������ʵ��Ʊ��в�ͬ�ķ��������á���ת�ķ糵��������ijþ�� F ���Ʊ�����������ͼ�����������ʢ١��ڡ������ηֱ���CuCl2��MgCO3��MgSO4������A��B��C��D��E���ηֱ����ڵ��ʡ�������ᡢ��Σ����糵����ÿƬ��Ҷ�֡������ڵ������������Ӧ������������F����
23��ijͬѧѧϰ�����з������ʵ��Ʊ��в�ͬ�ķ��������á���ת�ķ糵��������ijþ�� F ���Ʊ�����������ͼ�����������ʢ١��ڡ������ηֱ���CuCl2��MgCO3��MgSO4������A��B��C��D��E���ηֱ����ڵ��ʡ�������ᡢ��Σ����糵����ÿƬ��Ҷ�֡������ڵ������������Ӧ������������F���� 24������A��B��C��D��E��F���dz��л�ѧ�г��������ʣ�����������ͼ��ʾ��ת����ϵ���ش��������⣺
24������A��B��C��D��E��F���dz��л�ѧ�г��������ʣ�����������ͼ��ʾ��ת����ϵ���ش��������⣺ --��ԭ��
--��ԭ�� --��ԭ��
--��ԭ�� --��ԭ��
--��ԭ��



