��Ŀ����
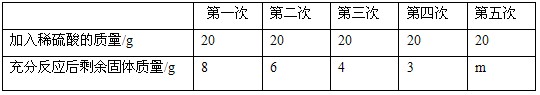
��������ͭ��ͭ�Ļ�������ɷֽ��з�����ȡ10g����Ʒ�������з���μ�����ͬ��������������ϡ���ᣬʹ֮��ַ�Ӧ��ÿ������ϡ�����������ʣ������������¼���±�������Ӧ�Ļ�ѧ����ʽ��CuO+H2SO4==CuSO4+H2O��
�Իش��������⣺
��1������������m��ֵΪ________��10g��Ʒ��CuO������Ϊ________g��
��2������������ϡ���������ʵ�����������
��3����������μ�ϡ���ᷴӦ��������Һ�����ʵ���������������ȷ��0.1%��
��1������������m��ֵΪ________��10g��Ʒ��CuO������Ϊ________g��
��2������������ϡ���������ʵ�����������
��3����������μ�ϡ���ᷴӦ��������Һ�����ʵ���������������ȷ��0.1%��
��1��3��7
��2���⣺���һ�η�Ӧ��H2SO4������Ϊx�������η�Ӧ������CuSO4������Ϊy
CuO+H2SO4==CuSO4+H2O
��80����98����160
(10-8)g��x
(10-4)g����������y
80��98=2g��x
x=2.45g
80��160=6g��y
y=12g
H2SO4��Һ��������������=2.45g��20g��100%=12.25%
��3�������μ�ϡ���ᷴӦ��������Һ��������������=12g��(60g+6g)��100% =18.2%
��������ϡ������������������Ϊ12.25%�������μ�ϡ���ᷴӦ��������Һ��������������Ϊ18.2%��
��2���⣺���һ�η�Ӧ��H2SO4������Ϊx�������η�Ӧ������CuSO4������Ϊy
CuO+H2SO4==CuSO4+H2O
��80����98����160
(10-8)g��x
(10-4)g����������y
80��98=2g��x
x=2.45g
80��160=6g��y
y=12g
H2SO4��Һ��������������=2.45g��20g��100%=12.25%
��3�������μ�ϡ���ᷴӦ��������Һ��������������=12g��(60g+6g)��100% =18.2%
��������ϡ������������������Ϊ12.25%�������μ�ϡ���ᷴӦ��������Һ��������������Ϊ18.2%��

��ϰ��ϵ�д�
�����Ŀ
| |||||||||||||||