��Ŀ����
��װ�ꡢװ�κ���д����ʱ�����õ�һ�֡���ۡ�����֪���֡���ۡ��������ֽ����γɵĺϽ��ĩ����λͬѧ��������̽��ʵ�飮
��ͬѧ��ȡ��������ۡ�����ȼ�ճ��У��þƾ��Ƽ��ȣ����֡���ۡ���ڣ�������ȼ�ճ�����ʢ��H2�ļ���ƿ�У���ɫ��ĩ�ָֻ����ɫ��
��ͬѧ����ȡ�������Թ��У�������ϡ���ᣬ�����������ʼ���к�ɫ���岻���ܽ⣮
��ͬѧ��ͨ������ʵ����һЩ���ݣ��ټ��������ۡ�����ϡ���ᷴӦ�Ľ��������ԭ������Ϊ65.4��
�ش��������⣺��д����ʵ�������Ӧ�Ļ�ѧ��Ӧ����ʽ�����жϷ�Ӧ���ͣ�
��ͬѧ��ʵ���н�۱�ڣ� �� ��Ӧ
��ͬѧ��ʵ���в������壺 �� ��Ӧ
�ڼ��衰��ۡ��Ǵ��Ļƽ��ڼס�����ͬѧ��ʵ����Ӧ�۲쵽���� ���� ��
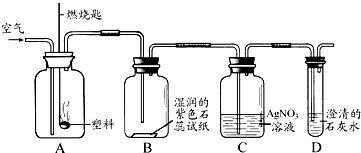
������ͬѧ��ʵ���У�����ϡ���ỻΪAgNO3��Һ�����ܷ�����Ӧ�Ļ�ѧ����ʽΪ �� ��
�ܱ�ͬѧ��ʵ���У�����Ӧ�ò�����һ�������� ��������ѡ�����ĸ��ţ���
A������ۡ������� B������ۡ����������ʣ����������
C����Ӧ�ų���������� D����Ӧ�ų������������ܶȣ�
��ͬѧ��ȡ��������ۡ�����ȼ�ճ��У��þƾ��Ƽ��ȣ����֡���ۡ���ڣ�������ȼ�ճ�����ʢ��H2�ļ���ƿ�У���ɫ��ĩ�ָֻ����ɫ��
��ͬѧ����ȡ�������Թ��У�������ϡ���ᣬ�����������ʼ���к�ɫ���岻���ܽ⣮
��ͬѧ��ͨ������ʵ����һЩ���ݣ��ټ��������ۡ�����ϡ���ᷴӦ�Ľ��������ԭ������Ϊ65.4��
�ش��������⣺��д����ʵ�������Ӧ�Ļ�ѧ��Ӧ����ʽ�����жϷ�Ӧ���ͣ�
��ͬѧ��ʵ���н�۱�ڣ�
��ͬѧ��ʵ���в������壺
�ڼ��衰��ۡ��Ǵ��Ļƽ��ڼס�����ͬѧ��ʵ����Ӧ�۲쵽����
������ͬѧ��ʵ���У�����ϡ���ỻΪAgNO3��Һ�����ܷ�����Ӧ�Ļ�ѧ����ʽΪ
�ܱ�ͬѧ��ʵ���У�����Ӧ�ò�����һ��������
A������ۡ������� B������ۡ����������ʣ����������
C����Ӧ�ų���������� D����Ӧ�ų������������ܶȣ�
����������Ŀ����Ϣ��֪��ȡ��������ۡ�����ȼ�ճ��У��þƾ��Ƽ��ȣ����֡���ۡ���ڣ�������ȼ�ճ�����ʢ��H2�ļ���ƿ�У���ɫ��ĩ�ָֻ����ɫ��˵���н���ͭ��ͭ��������Ӧ���ɺ�ɫ������ͭ��ͨ������ʵ����һЩ���ݣ��ټ��������ۡ�����ϡ���ᷴӦ�Ľ��������ԭ������Ϊ65.4����ˡ���ۡ��л��н���п��п�����ᷴӦ�����Ȼ�п����������ƽ���ɣ����衰��ۡ��Ǵ��Ļƽ��ڼס�����ͬѧ��ʵ����Ӧ�۲쵽������ۡ�ʼ�ղ���ɫ��������ʱ��������Ϊ��Ļ�ѧ���ʲ����ã�����п�����û����������û�ͭ��ͭҲ���û������йصļ�����ҪһЩȷ����ֵ��
����⣺������Ŀ����Ϣ��֪���н���ͭ��ͭ��������Ӧ���ɺ�ɫ������ͭ�����ڻ��Ϸ�Ӧ��ͨ������ʵ����һЩ���ݣ��ټ��������ۡ�����ϡ���ᷴӦ�Ľ��������ԭ������Ϊ65.4����ˡ���ۡ��л��н���п��п�����ᷴӦ�����Ȼ�п����������ƽ���ɣ������û���Ӧ���ʴ�Ϊ��2Cu+O2
2CuO�����ϣ�Zn+2HCl�TZnCl2+H2���� �û�
�ڼ��衰��ۡ��Ǵ��Ļƽ��ڼס�����ͬѧ��ʵ����Ӧ�۲쵽������ۡ�ʼ�ղ���ɫ��������ʱ��������Ϊ��Ļ�ѧ���ʲ����ã��ʴ�Ϊ������ۡ�ʼ�ղ���ɫ ������ʱ������
��п����������Ӧ��������п������ͭ����������Ӧ��������ͭ��������ƽ���ɣ��ʴ�Ϊ��Zn+2AgNO3�TZn��NO3��2+2Ag�� Cu+2AgNO3�TCu��NO3��2+2Ag
�ܱ�ͬѧ��ʵ���У�����Ӧ�ò�����һ�������ǣ�����ۡ�������������ۡ����������ʣ��������������Ӧ�ų��������������������Ҳ�ɣ��ʴ�Ϊ��ABC��ABD����ȫ�ԲŸ��֣�
| ||
�ڼ��衰��ۡ��Ǵ��Ļƽ��ڼס�����ͬѧ��ʵ����Ӧ�۲쵽������ۡ�ʼ�ղ���ɫ��������ʱ��������Ϊ��Ļ�ѧ���ʲ����ã��ʴ�Ϊ������ۡ�ʼ�ղ���ɫ ������ʱ������
��п����������Ӧ��������п������ͭ����������Ӧ��������ͭ��������ƽ���ɣ��ʴ�Ϊ��Zn+2AgNO3�TZn��NO3��2+2Ag�� Cu+2AgNO3�TCu��NO3��2+2Ag
�ܱ�ͬѧ��ʵ���У�����Ӧ�ò�����һ�������ǣ�����ۡ�������������ۡ����������ʣ��������������Ӧ�ų��������������������Ҳ�ɣ��ʴ�Ϊ��ABC��ABD����ȫ�ԲŸ��֣�
����������������ʵ��̽���⣬Ҳ�����˻�ѧ��Ӧ���͵��жϺͻ�ѧ����ʽ����д���й�ʵ�鷽������ƺͶ�ʵ�鷽�����������п����ȵ�֮һ�����ʵ�鷽��ʱ��Ҫע�������ٵ�ҩƷ����ķ��������ڶ�ʵ����Ʒ��������ۣ�Ҫ���������濼�ǣ�һ�Ƿ����Ƿ���У��ܷ�ﵽʵ��Ŀ�ģ�������Ƶķ������бȽϣ����ַ�������㣮��Ҫע�⻯ѧ����ʽ����д����ƽ����������Ҫ������ʵ�����У�

��ϰ��ϵ�д�
�����Ŀ