��Ŀ����
2009��2��20�����磬���ڳ���ˮ��ˮԴ�ܷ��������Ⱦ�������γ�����������Χ��ˮ������20�����������ܵ�Ӱ�죬ˮ�DZ������Ȼ��Դ���ڹ�ũҵ�������ճ����������ż���㷺�����ã���ͼΪ����ˮ����ˮ����ʾ��ͼ��
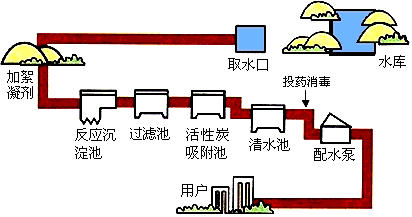
��1��Ϊ���ˮ���Ƿ�ﵽ����ˮ�ı�������________���ˮ�����ȣ�
��2����ͼ�������ڵĻ���̿���������ã������������˵Ⱦ������������õ�ˮ________������ˮ����ǡ����ǡ�����
��3������ˮ�����õ��������ж������ȣ�ClO2����Ư�ۡ���84����Һ���ȣ���ҵ����ȡƯ�۵Ļ�ѧ����ʽΪ2C12+2Ca��OH��2�TCaCl2+Ca��ClO��2+2H2O����ȡ��84����Һ���ǽ�����ͨ���ռ���Һ�еõ�����Ӧԭ����Ư�۵���ȡ���ƣ���д���÷�Ӧ�Ļ�ѧ����ʽ��________��
��4����ͥ�����п�����________����ijˮ����Ӳˮ������ˮ��
��5��������������ˮ���м��������Ҷ����������������ʣ���ʹ��Һ�����̵�________��
��6����Щ��ѧ��Ԥ�ԣ������������һ��ˮ������������ᡱ����仰��ʾ����Ӧ��������ˮ��Դ����ʶ��һ�ǽ�Լ��ˮ�����Ƿ�ֹˮ����Ⱦ�������һ����Լ��ˮ��������________��
�⣺��1���ⶨ��Һ�����ȿ���ʹ��pH��ֽ��pH�ƣ����Ա����Ϊ��pH��ֽ��pH�ƣ�
��2�������������˵Ⱦ������������õ�ˮ�к��п����Թ��壬���Ǵ���ˮ�����Ա����Ϊ�����ǣ�
��3���������������������ķ�Ӧ����֪������������������Ӧ�����Ȼ��ơ��������ƺ�ˮ�����Ա����Ϊ��Cl2+2NaOH�TNaCl+NaClO+H2O��
��4������Ӳˮ����ˮ����ʹ�÷���ˮ�����Ա����Ϊ������ˮ��
��5����ˮ�м����Ҷ����������������ʣ��ܽ���ˮ�����̵㣬���Ա����Ϊ�����ͣ�
��6����Լ��ˮ���Դ�С�����𣬱���������ˮ���������Ա����Ϊ������ˮ������
�������е�֪ʶ���м��𣬲ⶨ��Һ�����ȿ���ʹ��pH��ֽ��pH�ƣ������������˵Ⱦ������������õ�ˮ�к��п����Թ��壬���Ǵ���ˮ�������������������Ƶķ�Ӧ����Ǩ�Ƽ���д�����������������ķ�Ӧ������Ӳˮ����ˮ����ʹ�÷���ˮ����ˮ�м����Ҷ����������������ʣ��ܽ���ˮ�����̵㣬��Լ��ˮ���Դ�С�����𣬱���������ˮ������
���������⿼���˾���ˮ��֪ʶ����ɴ��⣬�����������е�֪ʶ�������ṩ����Ϣ���У�
��2�������������˵Ⱦ������������õ�ˮ�к��п����Թ��壬���Ǵ���ˮ�����Ա����Ϊ�����ǣ�
��3���������������������ķ�Ӧ����֪������������������Ӧ�����Ȼ��ơ��������ƺ�ˮ�����Ա����Ϊ��Cl2+2NaOH�TNaCl+NaClO+H2O��
��4������Ӳˮ����ˮ����ʹ�÷���ˮ�����Ա����Ϊ������ˮ��
��5����ˮ�м����Ҷ����������������ʣ��ܽ���ˮ�����̵㣬���Ա����Ϊ�����ͣ�
��6����Լ��ˮ���Դ�С�����𣬱���������ˮ���������Ա����Ϊ������ˮ������
�������е�֪ʶ���м��𣬲ⶨ��Һ�����ȿ���ʹ��pH��ֽ��pH�ƣ������������˵Ⱦ������������õ�ˮ�к��п����Թ��壬���Ǵ���ˮ�������������������Ƶķ�Ӧ����Ǩ�Ƽ���д�����������������ķ�Ӧ������Ӳˮ����ˮ����ʹ�÷���ˮ����ˮ�м����Ҷ����������������ʣ��ܽ���ˮ�����̵㣬��Լ��ˮ���Դ�С�����𣬱���������ˮ������
���������⿼���˾���ˮ��֪ʶ����ɴ��⣬�����������е�֪ʶ�������ṩ����Ϣ���У�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
2009��2��20��ij��������������������ˮʱ�ŵ��̱ǵ�ũҩζ������⣬��������ˮ����ˮԴ�ܵ�ij�������ŷŵķ���������Ⱦ���籽�Ӿ����ڷ�����������һ���ж������ʣ���Ƥ�����Ĥ��ǿ�ҵĸ�ʴ���ã��仯ѧʽΪC6H60������˵������ȷ���ǣ�������
| A�����������л��� | B�����ӷ�����̼���⡢��ԭ�ӵĸ�����Ϊ6��6��1 | C��������̼Ԫ�ص���������ԼΪ76.6% | D�����ӵ���Է�������Ϊ949 |
2009��2��20��6��20�����ҽ���ʡ�γ���������������������ˮʱ�ŵ��̱ǵ�ũҩζ������⣬���г�������ˮ����ˮԴ�ܵ��γ��б��»�������˾�ŷŵķ���������Ⱦ���籽�Ӿ����ڷ�����������һ���ж������ʣ���Ƥ�����Ĥ��ǿ�ҵĸ�ʴ���ã��仯ѧʽΪ��C6H6O��������˵����ȷ���ǣ�������
| A��������̼��������Ԫ�ص���������6��6��1 | B����������̼���⡢������ԭ�ӹ��ɵ� | C�����ӵ���Է�������Ϊ94g | D��������̼Ԫ�ص���������ԼΪ76.6% |
