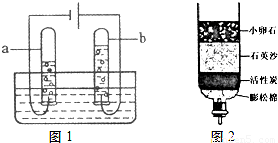
��Ŀ����
ˮ������֮Դ��Ҳ������������Դ������ѧ���Ļ�ѧ֪ʶ�ش��������⣺
��1����Ȼ���е�ˮ�����Ǵ�ˮ����ˮʱ�����������Ŀ����
��2������ȥˮ�в��������ʣ�����й��˲������ò��������в����������������
��3��������Ϊ����ˮ��Ӳ�Ȳ�ɱ��ˮ�в�ԭ����ɲ��õķ�����
��4����ͼΪ���ˮ��ʵ�飮
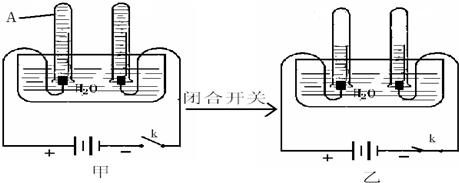
������A��������
�ڵ�Դ���رպ�ǰ���Ƚ�����A��������ͬ������ע��ˮ��Ŀ����
�۵���Դ���رպϺ�����Ӧ�Ļ�ѧ����ʽΪ
�ܸ�ʵ���ܹ�˵��ˮ������Ԫ������Ԫ����ɵ�����������
����ͼ��ʾ��������ʵ����ʵ������ϣ����ñ�����ͼ��������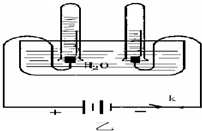
 ��
��
��1����Ȼ���е�ˮ�����Ǵ�ˮ����ˮʱ�����������Ŀ����
��������
��������
����2������ȥˮ�в��������ʣ�����й��˲������ò��������в����������������
����
����
����3��������Ϊ����ˮ��Ӳ�Ȳ�ɱ��ˮ�в�ԭ����ɲ��õķ�����
���
���
����4����ͼΪ���ˮ��ʵ�飮

������A��������
�Թ�
�Թ�
�����������ǵ�ľ����ȼ�յ�ľ��
�����ǵ�ľ����ȼ�յ�ľ��
��������A���ռ������壮�ڵ�Դ���رպ�ǰ���Ƚ�����A��������ͬ������ע��ˮ��Ŀ����
���ڱȽ���֧�Թܲ�����������ı���
���ڱȽ���֧�Թܲ�����������ı���
����ֹ�ռ������岻��
��ֹ�ռ������岻��
���۵���Դ���رպϺ�����Ӧ�Ļ�ѧ����ʽΪ
2H2O
2H2��+O2��
| ||
2H2O
2H2��+O2��
��
| ||
�ܸ�ʵ���ܹ�˵��ˮ������Ԫ������Ԫ����ɵ�����������
��ѧ��Ӧǰ��Ԫ������䣨�������غ㶨�ɻ�ԭ�ӵ����ࡢ��Ŀ���䣩
��ѧ��Ӧǰ��Ԫ������䣨�������غ㶨�ɻ�ԭ�ӵ����ࡢ��Ŀ���䣩
����ͼ��ʾ��������ʵ����ʵ������ϣ����ñ�����ͼ��������


��������1���������е�֪ʶ���з�������������ˮ�γɵĽ��������������IJ����Թ���С�������γɵĴ�������ڳ�����
��2������ʱ�������������������ã�
��3��Ӳˮ�к��н϶�Ŀ����Ը�þ����������ֽ����ɲ����Ը�þ�����ϸ�����������ʧȥ���ԣ�
��4�����ˮʱ�������ɵ������������ɵ����������ǵ������Ϊ1��2��
��2������ʱ�������������������ã�
��3��Ӳˮ�к��н϶�Ŀ����Ը�þ����������ֽ����ɲ����Ը�þ�����ϸ�����������ʧȥ���ԣ�
��4�����ˮʱ�������ɵ������������ɵ����������ǵ������Ϊ1��2��
����⣺��1����������ˮ�γɵĽ��������������IJ����Թ���С�������γɵĴ�������ڳ������������������
��2������ʱ�������������������ã����������
��3��Ӳˮ�к��н϶�Ŀ����Ը�þ����������ֽ����ɲ����Ը�þ�����Ӳˮ�������Բ��ü��ȵķ�����ϸ�����������ʧȥ���ԣ������У�
��4�����ˮʱ�������ɵ������������ɵ����������ǵ������Ϊ1��2��
������A���������Թܣ��Թ�A���ռ��������������������Կ��ô����ǵ�ľ����ȼ�յ�ľ����������A���ռ������壮
�ڵ�Դ���رպ�ǰ���Ƚ�����A��������ͬ������ע��ˮ��Ŀ���DZ��ڱȽ���֧�Թܲ�����������ı����ͷ�ֹ�ռ������岻����
�۵���Դ���رպϺ�ˮͨ��ֽ�����������������������Ӧ�Ļ�ѧ����ʽΪ2H2O
2H2��+O2����
�ܸ�ʵ���ܹ�˵��ˮ������Ԫ������Ԫ����ɵ����������ǻ�ѧ��Ӧǰ��Ԫ������� ���������غ㶨�ɻ�ԭ�ӵ����ࡢ��Ŀ���䣩��
����ͼ��ʾ��������ʵ����ʵ������ϣ���ȷ��ͼΪ�� ��
��
�ʴ�Ϊ�����Թܣ������ǵ�ľ����ȼ�յ�ľ����
�ڱ��ڱȽ���֧�Թܲ�����������ı�������ֹ�ռ������岻����
��2H2O
2H2��+O2����
�ܻ�ѧ��Ӧǰ��Ԫ������� ���������غ㶨�ɻ�ԭ�ӵ����ࡢ��Ŀ���䣩��
�� ��
��
��2������ʱ�������������������ã����������
��3��Ӳˮ�к��н϶�Ŀ����Ը�þ����������ֽ����ɲ����Ը�þ�����Ӳˮ�������Բ��ü��ȵķ�����ϸ�����������ʧȥ���ԣ������У�
��4�����ˮʱ�������ɵ������������ɵ����������ǵ������Ϊ1��2��
������A���������Թܣ��Թ�A���ռ��������������������Կ��ô����ǵ�ľ����ȼ�յ�ľ����������A���ռ������壮
�ڵ�Դ���رպ�ǰ���Ƚ�����A��������ͬ������ע��ˮ��Ŀ���DZ��ڱȽ���֧�Թܲ�����������ı����ͷ�ֹ�ռ������岻����
�۵���Դ���رպϺ�ˮͨ��ֽ�����������������������Ӧ�Ļ�ѧ����ʽΪ2H2O
| ||
�ܸ�ʵ���ܹ�˵��ˮ������Ԫ������Ԫ����ɵ����������ǻ�ѧ��Ӧǰ��Ԫ������� ���������غ㶨�ɻ�ԭ�ӵ����ࡢ��Ŀ���䣩��
����ͼ��ʾ��������ʵ����ʵ������ϣ���ȷ��ͼΪ��
 ��
���ʴ�Ϊ�����Թܣ������ǵ�ľ����ȼ�յ�ľ����
�ڱ��ڱȽ���֧�Թܲ�����������ı�������ֹ�ռ������岻����
��2H2O
| ||
�ܻ�ѧ��Ӧǰ��Ԫ������� ���������غ㶨�ɻ�ԭ�ӵ����ࡢ��Ŀ���䣩��
��
 ��
�����������⿼����ˮ�ľ����Լ������غ㶨�ɵ�Ӧ�ã���ɴ��⣬�����������е�֪ʶ���У�

��ϰ��ϵ�д�
 Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д� ������������Ծ�ϵ�д�
������������Ծ�ϵ�д� �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д�
�����Ŀ
 ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺
ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺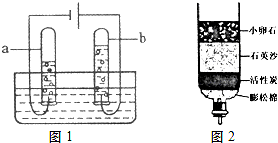 ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺
ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺