��Ŀ����
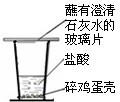 ��������֪���������ǵ���Ҫ�ɷ���̼��ƣ�ijͬѧΪ����֤�����ǵ���Ҫ�ɷ���̼��ƣ��������ͼ��ʵ�飮
��������֪���������ǵ���Ҫ�ɷ���̼��ƣ�ijͬѧΪ����֤�����ǵ���Ҫ�ɷ���̼��ƣ��������ͼ��ʵ�飮
��1��ʵ������п�����������______��
��2��С����Ϊ��ʵ�鷽����������������֤�����ǵ���Ҫ�ɷ����ڸ��Σ�������ȡ��Ӧ����ϲ���Һ����______������ĸ����
A��Na2CO3��Һ��B��AgNO3��Һ��C��NaCl��Һ����D��Ca��OH��2��Һ
��3�������������ᷴӦ�Ļ�ѧ����ʽΪ______��
�⣺
��1�������ǵ���Ҫ�ɷ���̼��ƣ����������ᷴӦ���ɶ�����̼���壬���ɵĶ�����̼�����������ʯ��ˮ����ǣ���ʵ������Ϊ��������٣������ݷų�������ʯ��ˮ����ǣ�
��2��A��̼������Ӻ����ӻ�����̼��Ƴ��������Լ��𣬹�A��ȷ��
B�����е������ζ�����ˮ�����ܼ��𣬹�B����
C�������Ӻ������γɵ��Ȼ���û�������ܼ��𣬹�C����
D���÷�Ӧû�������ᷴӦ����D����
��ѡA��
��3������1����2����֪�����ǵ���Ҫ�ɷ�Ϊ̼��ƣ������ᷴӦ�Ļ�ѧ������ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
�ʴ�Ϊ��
��1��������٣������ݷų�������ʯ��ˮ����ǣ�
��2��A��
��3��CaCO3+2HCl=CaCl2+H2O+CO2����
��������֤�����dzɷ���̼��ƣ�̼���ο������ᷴӦ����ʹ����ʯ��ˮ����ǵ����������̼�����Լ���̼�������õ��Լ��������ʯ��ˮ�����μ�����̼���������飮
���������⿼����̼���εļ��飬����̼�������õ��Լ��������ʯ��ˮ����ɴ��⣬�����������е�֪ʶ���У�
��1�������ǵ���Ҫ�ɷ���̼��ƣ����������ᷴӦ���ɶ�����̼���壬���ɵĶ�����̼�����������ʯ��ˮ����ǣ���ʵ������Ϊ��������٣������ݷų�������ʯ��ˮ����ǣ�
��2��A��̼������Ӻ����ӻ�����̼��Ƴ��������Լ��𣬹�A��ȷ��
B�����е������ζ�����ˮ�����ܼ��𣬹�B����
C�������Ӻ������γɵ��Ȼ���û�������ܼ��𣬹�C����
D���÷�Ӧû�������ᷴӦ����D����
��ѡA��
��3������1����2����֪�����ǵ���Ҫ�ɷ�Ϊ̼��ƣ������ᷴӦ�Ļ�ѧ������ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
�ʴ�Ϊ��
��1��������٣������ݷų�������ʯ��ˮ����ǣ�
��2��A��
��3��CaCO3+2HCl=CaCl2+H2O+CO2����
��������֤�����dzɷ���̼��ƣ�̼���ο������ᷴӦ����ʹ����ʯ��ˮ����ǵ����������̼�����Լ���̼�������õ��Լ��������ʯ��ˮ�����μ�����̼���������飮
���������⿼����̼���εļ��飬����̼�������õ��Լ��������ʯ��ˮ����ɴ��⣬�����������е�֪ʶ���У�

��ϰ��ϵ�д�
�����Ŀ
 20����������֪���������ǵ���Ҫ�ɷ���̼��ƣ�ijͬѧΪ����֤�����ǵ���Ҫ�ɷ���̼��ƣ��������ͼ��ʵ�飮
20����������֪���������ǵ���Ҫ�ɷ���̼��ƣ�ijͬѧΪ����֤�����ǵ���Ҫ�ɷ���̼��ƣ��������ͼ��ʵ�飮 18����������֪���������ǵ���Ҫ�ɷ���̼��ƣ�ijͬѧΪ����֤�����ǵ���Ҫ�ɷ���̼��ƣ��������ͼ��ʵ�飮
18����������֪���������ǵ���Ҫ�ɷ���̼��ƣ�ijͬѧΪ����֤�����ǵ���Ҫ�ɷ���̼��ƣ��������ͼ��ʵ�飮 6����������֪���������ǵ���Ҫ�ɷ���̼��ƣ�ijͬѧΪ����֤�����ǵ���Ҫ�ɷ���̼��ƣ��������ͼ��ʵ�飮
6����������֪���������ǵ���Ҫ�ɷ���̼��ƣ�ijͬѧΪ����֤�����ǵ���Ҫ�ɷ���̼��ƣ��������ͼ��ʵ�飮
