��Ŀ����
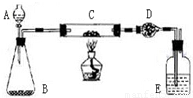
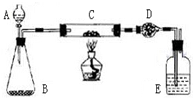 ij����С�����̿�ۣ�������������ͭ�Ļ���������ͼװ�ã��Ի�õ�ͭ�ۣ���̿����Ʒ����ʵ�飮ͼ������̨��װ������ȥ��������������������ʵ�鱨�森
ij����С�����̿�ۣ�������������ͭ�Ļ���������ͼװ�ã��Ի�õ�ͭ�ۣ���̿����Ʒ����ʵ�飮ͼ������̨��װ������ȥ��������������������ʵ�鱨�森
��һ��ʵ��Ŀ�ģ�________��
������ʵ����Ʒ����������ƽ����Һ©������ƿ��Ӳ�����ܡ�����ܡ��ƾ��ơ�ϴ��ƿ�ȣ�
ʵ��ҩƷ�����ɫͭ�ۣ���̿����Ʒ������������Һ���������̡���ʯ�ң�Ũ����ȣ�
������ʵ�����ݣ�
| ʵ����� | ʵ������ | �йػ�ѧ����ʽ |
| ��C�м�����Ʒ�걾W�ˣ�D��װ��ҩƷ����Ϊm1�ˣ����Ӻ�������������� | \ | \ |
| ��A�Ļ����������μ�Һ�� | ________ | ________ |
| ��C���м��ȣ���C��ҩƷ��ַ�Ӧ�ر�A�Ļ�����ֹͣ���� | ________ | ________ |
| ��ȴ����D������Ϊm2�� | \ | \ |
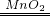 2H2O+O2�� C�еĺ��ɫ��ĩ��ɺ�ɫ 2Cu+O2
2H2O+O2�� C�еĺ��ɫ��ĩ��ɺ�ɫ 2Cu+O2 2CuO
2CuOC+O2
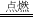 CO2
CO2 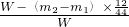
��������һ������ʵ��װ������ͼ��ʵ�鲽���ƶ�ʵ��Ŀ��
����������ÿ��װ���е�ҩƷ�����÷���������ʽ
���ģ����ݶ�����̼��̼Ԫ�ص�����������������ȥ̼����������ͭ���������Ӷ����ͭ������������
��𣺣�һ����ʵ��װ������ͼ���Կ���Bװ����˫��ˮ��������Cװ����ͭ��̼�ֱ���������Ӧ��̼ת���ɶ�����̼��Dװ�����գ�Dװ�����ص������������ɵĶ�����̼���������ɴ˿ɼ������Ʒ��̼���������Ӷ������Ʒ��ͭ����������
������B����˫��ˮ�����������BE�п����������������ݲ�������ѧ����ʽΪ2H2O2
 2H2O+O2��
2H2O+O2��C��ͭ�������ڼ�����������������ͭ��C�������ڵ�ȼ���������ɶ�����̼����˿ɿ���C�к�ɫ��ĩ��ɺ�ɫ����ѧ����ʽΪ2Cu+O2
 2CuO��C+O2
2CuO��C+O2 CO2
CO2���ģ�Dװ�����ص�������������̼������Ϊm2-m1��������̼��̼Ԫ�ص�����Ϊ��m2-m1����
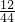 ������Ʒ��ͭ����������Ϊ
������Ʒ��ͭ����������Ϊ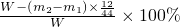
�ʴ�Ϊ����һ���ⶨͭ����Ʒ��ͭ����������
������
| B��E�������ݲ��� | 2H2O2 2H2O+O2�� 2H2O+O2�� |
| C�еĺ��ɫ��ĩ��ɺ�ɫ | 2Cu+O2 2CuO 2CuOC+O2  CO2 CO2 |
 ��100%
��100%����������ʵ��װ�ü�ҩƷ�������ã�����Ԫ�������غ�����������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�ij����С�����̿��(����)������ͭ�Ļ���������ͼ��ʾװ�ã��Ի�õ�ͭ��(��̿)��Ʒ����ʵ�顣ͼ������̨��װ������ȥ��������������������ʵ�鱨�档
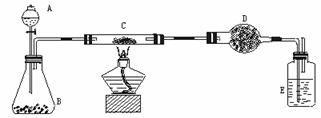
(һ)ʵ��Ŀ�ģ� ��
(��)ʵ����Ʒ��
��������ƽ����Һ©������ƿ��Ӳ�ʲ����ܡ�����ܡ��ƾ��ơ�ϴ��ƿ�ȡ�
ҩƷ�����ɫ(��̿)��Ʒ������������Һ���������̡���ʯ�ң������������ƺ������ƵĻ�����Ũ����ȡ�
(��)ʵ�����ݣ�
| ʵ�鲽�� | ʵ������ | �йػ�ѧ����ʽ |
| ��C�м�����Ʒ��ĩW g��D��װ��ҩƷ����Ϊm1 g�����Ӻ������� | ||
| ��A�������������������μ���Һ�� | ||
| ��C���м��ȡ���C��ҩƷ��ַ�Ӧ�ر�A�Ļ�����ֹͣ���ȡ� | ||
| ��ȴ����D������Ϊm2 g�� |
(��)���㣺��Ʒ��ͭ������������ (�ú�����m1��m2�Ĵ���ʽ��ʾ)
(��)��������ۣ�
ʵ����ɺ���ʦ����˵��������ʵ����ƣ���ʹC�з�Ӧ��ȫ��D��������ȫ��Ҳ����ó���ȷ�Ľ���������ۣ���ͬѧ�����B��C֮�����һ��װ�á��ٴ�ʵ��õ��˽���ȷ�Ľ������ô��ԭ��ʵ������õ�ͭ����������ƫС��ԭ������� ����B��C֮������װ�ÿ����� ������ʢ�ŵ�ҩƷ�� ��
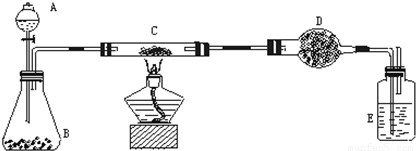
��1��ʵ��Ŀ�ģ�______��
��2��ʵ����Ʒ��
��������ƽ����Һ©������ƿ��Ӳ�ʲ����ܡ�����ܡ��ƾ��ơ�ϴ��ƿ�ȣ�
ҩƷ�����ɫ����̿����Ʒ������������Һ���������̡���ʯ�ң������������ƺ������ƵĻ�����Ũ����ȣ�
��3��ʵ�����ݣ�
| ʵ�鲽�� | ʵ������ | �йػ�ѧ����ʽ |
| ��C�м�����Ʒ��ĩW g��D��װ��ҩƷ����Ϊm1 g�����Ӻ������� | ||
| ��A�������������������μ���Һ�� | ||
| ��C���м��ȣ���C��ҩƷ��ַ�Ӧ�� ��A�Ļ�����ֹͣ���ȣ� | ||
| ��ȴ����D������Ϊm2 g�� |
��5����������ۣ�ʵ����ɺ���ʦ����˵��������ʵ����ƣ���ʹC�з�Ӧ��ȫ��D��������ȫ��Ҳ����ó���ȷ�Ľ���������ۣ���ͬѧ�����B��C֮�����һ��װ�ã��ٴ�ʵ��õ��˽���ȷ�Ľ������ô��ԭ��ʵ������õ�ͭ����������ƫС��ԭ�������______����B��C֮������װ�ÿ�����______������ʢ�ŵ�ҩƷ��______��
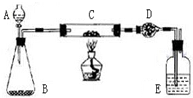
 ij����С�����̿�ۣ�������������ͭ�Ļ���������ͼװ�ã��Ի�õ�ͭ�ۣ���̿����Ʒ����ʵ�飮ͼ������̨��װ������ȥ��������������������ʵ�鱨�森
ij����С�����̿�ۣ�������������ͭ�Ļ���������ͼװ�ã��Ի�õ�ͭ�ۣ���̿����Ʒ����ʵ�飮ͼ������̨��װ������ȥ��������������������ʵ�鱨�森��һ��ʵ��Ŀ�ģ�______��
������ʵ����Ʒ����������ƽ����Һ©������ƿ��Ӳ�����ܡ�����ܡ��ƾ��ơ�ϴ��ƿ�ȣ�
ʵ��ҩƷ�����ɫͭ�ۣ���̿����Ʒ������������Һ���������̡���ʯ�ң�Ũ����ȣ�
������ʵ�����ݣ�
| ʵ����� | ʵ������ | �йػ�ѧ����ʽ |
| ��C�м�����Ʒ�걾W�ˣ�D��װ��ҩƷ����Ϊm1�ˣ����Ӻ�������������� | \ | \ |
| ��A�Ļ����������μ�Һ�� | ______ | ______ |

 ij����С�����̿�ۣ�������������ͭ�Ļ���������ͼװ�ã��Ի�õ�ͭ�ۣ���̿����Ʒ����ʵ�飮ͼ������̨��װ������ȥ��������������������ʵ�鱨�森
ij����С�����̿�ۣ�������������ͭ�Ļ���������ͼװ�ã��Ի�õ�ͭ�ۣ���̿����Ʒ����ʵ�飮ͼ������̨��װ������ȥ��������������������ʵ�鱨�森