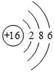
��Ŀ����
��1������ѧʹ�������ˮ���塣������β������װ����ʹijЩ�ж�����ת��Ϊ�����壺2NO+2CO��N2+2CO2���ڸ÷�Ӧ�л��ϼ۱仯��Ԫ��Ϊ �� ����дԪ�����ƣ�����Ӧ���漰�������У� �� �ǹ�����õ�ԭ�ϣ� �� ����Ѫ�쵰��ϣ�������һ�������ǿ����к�����ߵģ����������ԼΪ �� ��
��2����H��C��O��Ca����Ԫ���У�ѡ���ʵ���Ԫ����ɷ�������Ҫ������ʡ�����д��ѧʽ��
�� ��������Ҫ�ɷ� ����������ܼ�
�� �ɱ�����ֱ�����յ��� �����ܶ�������ʯ����Ҫ�ɷ�
��1����Ԫ�أ�������̼��һ����̼��78%��
��2��CH4��H2O��C6H12O6��CaCO3
���������������1�����ݡ��ڻ������У��������ϼ۵Ĵ�����Ϊ�㡱��ԭ����֪��Ϊ-2�ۣ���ôһ�������е�Ԫ�صĻ��ϼ�Ϊ-2�ۣ������ǵ��ʣ�Ԫ�صĻ��ϼ�Ϊ0��������������ÿ����е�ˮ�Ͷ�����̼Ϊԭ�ϣ��õ��л����������һ����̼�ж�������Ѫ�쵰��ϣ��Ӷ���ֹ������Ѫ�쵰��ϣ������к��������ǵ�����ռ78%��
��2����������Ҫ�ɷ��Ǽ��飻������ܼ���ˮ���ɱ�����ֱ�����յ����������ǣ��ܶ�������ʯ����Ҫ�ɷ���̼��ơ�
���㣺��ѧʽ����д�����ʵ�����

 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д� �� P��ֵΪ___________��
�� P��ֵΪ___________��