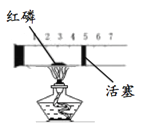
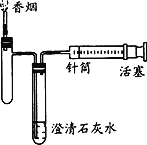
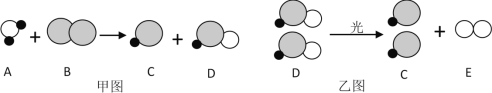
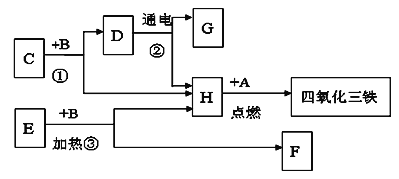
��Ŀ����
����Ŀ��ȱ���ܵ��¶�ͯ�������������Ͳ���С��ͬѧ��ʳ���������Ԫ�ص������㣬ÿ����Ҫ����2Ƭij�ָ�Ƭ���ø�Ƭ��ǩ�IJ���������ͼ��ʾ�������Ƭ��ֻ��̼��ƺ��и�Ԫ�أ�������㣺

��1��̼����и�Ԫ�ص������ȣ�
��2��̼����и�Ԫ�ص�����������
��3��С��ͬѧÿ��Ӹ�Ƭ�������Ԫ�ص�������
��4����С�����ú�ţ�̣�ÿ100mlţ���к��ơ�0.10g��������ƣ�ÿ��������Ҫ�ȶ��ٺ���ţ��
���𰸡���1���ƣ�̼���� =10:3:12��2��40����3��0.6�ˣ�4��600ml
��������
�����������1��̼����иơ�̼����Ԫ�ص�������Ϊ��40:12:16��3=10:3:12��
��2��̼����и�Ԫ�ص���������Ϊ��40/100��100%=40%
��3��ÿ��Ӹ�Ƭ�������Ԫ�ص�������2��0.75g��40%=0.6g
��4����С�����ú�ţ�̣�ÿ100mlţ���к��ơ�0.10g��������ƣ�ÿ��������Ҫ��ţ�̵����Ϊ��0.6g/0.10g/100ml=600ml

��ϰ��ϵ�д�
 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д� ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д�
�����Ŀ