��Ŀ����
��7�֣��������ִ������в���ȱ�ٵĴ������ߡ���ش��������⣺

��1��������·�еĵ��ߴ���ͭ�Ƶ����������˽���ͭ����չ�Ժ�____________�ԡ�
��2�����������������Ʒ�У������л��ϳɲ��ϵ����������ͬ____ ______��
a?������� b?�������� c?����̥ d?��ë����
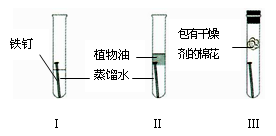
��3�����ڳ�ʪ�Ŀ�����������ʴ��
�������������ᣬ�����ӻ���������ʴ�������ԭ���Ǹ���____________��ˮ��
������ǰ�轫����Ʒ����ϡ�����г��⣬������Ҫ�ɷ���Fe2O3���۲쵽��
Һ��ƣ�����ɫ�����ݳ�����Һ��Ʒ�Ӧ�Ļ�ѧ����ʽ��_____________ ��
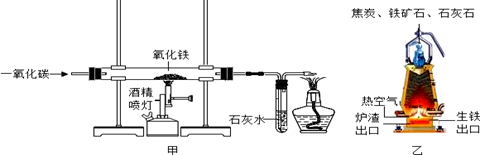
��4�������������������Ʒ�DZ���������Դ����Ч;������ͼ��ʾ�ķ�������Fe2O3������Լ��80%(����20%Ϊ��)���ң����պ��ڹ�ҵ�ϳ���һ����̼���仹ԭ����Ӧ�Ļ�ѧ����ʽ��_______________ ��


��1��������·�еĵ��ߴ���ͭ�Ƶ����������˽���ͭ����չ�Ժ�____________�ԡ�
��2�����������������Ʒ�У������л��ϳɲ��ϵ����������ͬ____ ______��
a?������� b?�������� c?����̥ d?��ë����
��3�����ڳ�ʪ�Ŀ�����������ʴ��
�������������ᣬ�����ӻ���������ʴ�������ԭ���Ǹ���____________��ˮ��
������ǰ�轫����Ʒ����ϡ�����г��⣬������Ҫ�ɷ���Fe2O3���۲쵽��
Һ��ƣ�����ɫ�����ݳ�����Һ��Ʒ�Ӧ�Ļ�ѧ����ʽ��_____________ ��
��4�������������������Ʒ�DZ���������Դ����Ч;������ͼ��ʾ�ķ�������Fe2O3������Լ��80%(����20%Ϊ��)���ң����պ��ڹ�ҵ�ϳ���һ����̼���仹ԭ����Ӧ�Ļ�ѧ����ʽ��_______________ ��

��1��?���� ��2��?c ��3��?�ٿ���������? �� Fe2O3 + 6HCl ="=" 2FeCl3 + 3H2O
��4�� Fe2O3 + 3CO ���� 2Fe + 3CO2?
��4�� Fe2O3 + 3CO ���� 2Fe + 3CO2?
�����������1��ͭ���е����ԣ��������ߣ�
��2���ϳɲ��ϰ������ϡ��ϳ���ά���ϳ���
��3���������������ˮ����������ֹ��������������ƻ�������������������������ᷴӦ�����Ȼ�����ˮ��
��4��һ����̼��ԭ�������������Ͷ�����̼��

��ϰ��ϵ�д�
�����Ŀ