��Ŀ����
��1��ʵ�����г�������غͶ������̼�����������п����ϡ���ᷴӦ��������ʯ��ʯ��ϡ�����ƶ�����̼���壮 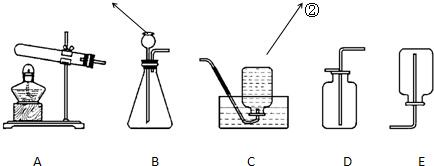
д��������������Ƣ�______����______
��������ҩƷѡ���ʵ������巢�����ռ�װ�������±���������ĸ��
| ���� | ����װ�� | �ռ�װ�� |
| O2 | ||
| H2 | ||
| CO2 |

������B��������______��
������E�������______��
��������C�IJ������з����������������ΪWg�������������ȼ�պ�������D����a g����Wg�����������к���Ԫ�ص�����Ϊ______g����������Ϊ������ʽ��
����װ����û����������B����ʹ��������������Ԫ�ص�����������______���ƫС������ƫ������Ӱ�족��֮һ����
�⣺��1���ٳ���©�����ڼ���ƿ
��2��װ��B��װ����Ũ���ᣬŨ�������ڳ�ˮ������
��3��װ��E��װ���dz����ʯ��ˮ�����������������̼�ģ�������̼��ʹ�����ʯ��ˮ����ǣ�
��4�������������ȼ�պ�Ũ���������ˮ�ԣ���D���ص���������W g����������ȼ�����ɵ�ˮ��������װ��D����a g���������غ㶨�ɣ�ˮ����Ԫ�ؼ�������Ʒ�е���Ԫ�أ�����W g�����������к���Ԫ�ص�����Ϊ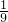 ag��
ag��
��5��������װ����û����������B����ʹ�����е�ˮ�������װ��D���Ӷ�ʹʵ���õ�ˮ����������������Ʒȼ�����ɵ�ˮ���ʽ�ʹ��������������Ԫ�ص�����������ƫ��
�ʴ�Ϊ��
��1���ٳ���©�����ڼ���ƿ
��2���ٳ�ȥ�����е�ˮ�������ڳ���ʯ��ˮ����ǣ���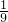 a����ƫ��
a����ƫ��
��������1�����ݷ���װ�ú��ռ�װ�õ�ѡ�����ݽ��з������
��2����ij�����ϴ������Ԫ�ؽ��з���̽�����ú�������Ϣ����Ͽα�֪ʶ����Ԫ�ص�����ȼ�ղ���ˮ����̼Ԫ�ص�����ȼ�����ɶ�����̼������֪ʶ�ٽ������ʵ������⣮����õ����������غ㶨�ɺͻ�ѧʽ����ijԪ�ص�����������
������������ʵ��̽���⣬������װ�õ�ѡ�����ݼ���������̼Ԫ�غ���Ԫ�ص���֤���̺��ݻ�ѧʽ�ļ��㣬�ۺ��Լ�ǿ��ֻҪ���巴Ӧ��ʵ�ʽ������⣮
��2��װ��B��װ����Ũ���ᣬŨ�������ڳ�ˮ������
��3��װ��E��װ���dz����ʯ��ˮ�����������������̼�ģ�������̼��ʹ�����ʯ��ˮ����ǣ�
��4�������������ȼ�պ�Ũ���������ˮ�ԣ���D���ص���������W g����������ȼ�����ɵ�ˮ��������װ��D����a g���������غ㶨�ɣ�ˮ����Ԫ�ؼ�������Ʒ�е���Ԫ�أ�����W g�����������к���Ԫ�ص�����Ϊ
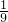 ag��
ag����5��������װ����û����������B����ʹ�����е�ˮ�������װ��D���Ӷ�ʹʵ���õ�ˮ����������������Ʒȼ�����ɵ�ˮ���ʽ�ʹ��������������Ԫ�ص�����������ƫ��
�ʴ�Ϊ��
��1���ٳ���©�����ڼ���ƿ
| ���� | ����װ�� | �ռ�װ�� |
| O2 | A | C��D |
| H2 | B | C��E |
| CO2 | B | D |
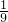 a����ƫ��
a����ƫ����������1�����ݷ���װ�ú��ռ�װ�õ�ѡ�����ݽ��з������
��2����ij�����ϴ������Ԫ�ؽ��з���̽�����ú�������Ϣ����Ͽα�֪ʶ����Ԫ�ص�����ȼ�ղ���ˮ����̼Ԫ�ص�����ȼ�����ɶ�����̼������֪ʶ�ٽ������ʵ������⣮����õ����������غ㶨�ɺͻ�ѧʽ����ijԪ�ص�����������
������������ʵ��̽���⣬������װ�õ�ѡ�����ݼ���������̼Ԫ�غ���Ԫ�ص���֤���̺��ݻ�ѧʽ�ļ��㣬�ۺ��Լ�ǿ��ֻҪ���巴Ӧ��ʵ�ʽ������⣮

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��1��ʵ�����г�������غͶ������̼�����������п����ϡ���ᷴӦ��������ʯ��ʯ��ϡ�����ƶ�����̼���壮
д��������������Ƣ� ��
��������ҩƷѡ���ʵ������巢�����ռ�װ�������±���������ĸ��
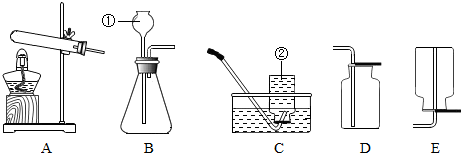
��2�����ڴ���ʹ��һ�������Ϸ������ɵġ���ɫ��Ⱦ�����ѳ�Ϊһ�����ص�������⣮ij��ѧ�о�С���ͬѧ����ij�����ϴ�����ɽ��з���̽����������ʾ������ֻ��C��H����Ԫ�أ��������������ͼ��ʾ��ʵ��װ�ã�ʹ�����������ڴ�����ȼ�գ��۲�ʵ���������й����ݣ�����Ԫ�غ�����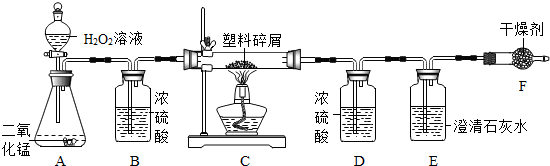
������B�������� ��
������E������� ��
��������C�IJ������з����������������ΪWg�������������ȼ�պ�������D����a g����Wg�����������к���Ԫ�ص�����Ϊ g����������Ϊ������ʽ��
����װ����û����������B����ʹ��������������Ԫ�ص����������� ���ƫС������ƫ������Ӱ�족��֮һ����

д��������������Ƣ�
��������ҩƷѡ���ʵ������巢�����ռ�װ�������±���������ĸ��
| ���� | ����װ�� | �ռ�װ�� |
| O2 | ||
| H2 | ||
| CO2 |

������B��������
������E�������
��������C�IJ������з����������������ΪWg�������������ȼ�պ�������D����a g����Wg�����������к���Ԫ�ص�����Ϊ
����װ����û����������B����ʹ��������������Ԫ�ص�����������
��1��ʵ�����г�������غͶ������̼�����������п����ϡ���ᷴӦ��������ʯ��ʯ��ϡ�����ƶ�����̼���壮 
д��������������Ƣ�______ ��______
��������ҩƷѡ���ʵ������巢�����ռ�װ�������±���������ĸ��
��2�����ڴ���ʹ��һ�������Ϸ������ɵġ���ɫ��Ⱦ�����ѳ�Ϊһ�����ص�������⣮ij��ѧ�о�С���ͬѧ����ij�����ϴ�����ɽ��з���̽����������ʾ������ֻ��C��H����Ԫ�أ��������������ͼ��ʾ��ʵ��װ�ã�ʹ�����������ڴ�����ȼ�գ��۲�ʵ���������й����ݣ�����Ԫ�غ�����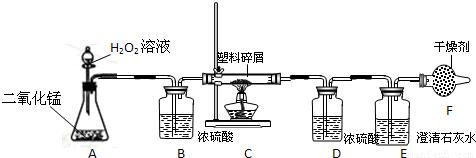
������B��������______��
������E�������______��
��������C�IJ������з����������������ΪWg�������������ȼ�պ�������D����a g����Wg�����������к���Ԫ�ص�����Ϊ______g����������Ϊ������ʽ��
����װ����û����������B����ʹ��������������Ԫ�ص�����������______���ƫС������ƫ������Ӱ�족��֮һ����

д��������������Ƣ�______ ��______
��������ҩƷѡ���ʵ������巢�����ռ�װ�������±���������ĸ��
| ���� | ����װ�� | �ռ�װ�� |
| O2 | ||
| H2 | ||
| CO2 |

������B��������______��
������E�������______��
��������C�IJ������з����������������ΪWg�������������ȼ�պ�������D����a g����Wg�����������к���Ԫ�ص�����Ϊ______g����������Ϊ������ʽ��
����װ����û����������B����ʹ��������������Ԫ�ص�����������______���ƫС������ƫ������Ӱ�족��֮һ����
��2012?������һģ����1��ʵ�����г�������غͶ������̼�����������п����ϡ���ᷴӦ��������ʯ��ʯ��ϡ�����ƶ�����̼���壮 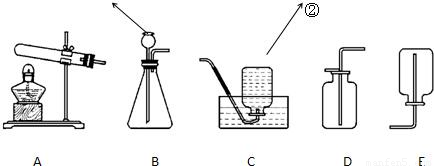
д��������������Ƣ�______ ��______
��������ҩƷѡ���ʵ������巢�����ռ�װ�������±���������ĸ��
��2�����ڴ���ʹ��һ�������Ϸ������ɵġ���ɫ��Ⱦ�����ѳ�Ϊһ�����ص�������⣮ij��ѧ�о�С���ͬѧ����ij�����ϴ�����ɽ��з���̽����������ʾ������ֻ��C��H����Ԫ�أ��������������ͼ��ʾ��ʵ��װ�ã�ʹ�����������ڴ�����ȼ�գ��۲�ʵ���������й����ݣ�����Ԫ�غ�����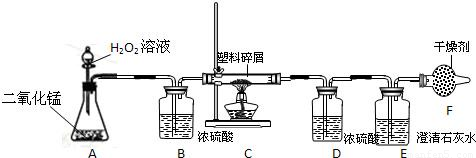
������B��������______��
������E�������______��
��������C�IJ������з����������������ΪWg�������������ȼ�պ�������D����a g����Wg�����������к���Ԫ�ص�����Ϊ______g����������Ϊ������ʽ��
����װ����û����������B����ʹ��������������Ԫ�ص�����������______���ƫС������ƫ������Ӱ�족��֮һ����

д��������������Ƣ�______ ��______
��������ҩƷѡ���ʵ������巢�����ռ�װ�������±���������ĸ��
| ���� | ����װ�� | �ռ�װ�� |
| O2 | ||
| H2 | ||
| CO2 |

������B��������______��
������E�������______��
��������C�IJ������з����������������ΪWg�������������ȼ�պ�������D����a g����Wg�����������к���Ԫ�ص�����Ϊ______g����������Ϊ������ʽ��
����װ����û����������B����ʹ��������������Ԫ�ص�����������______���ƫС������ƫ������Ӱ�족��֮һ����