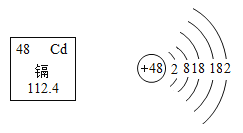��Ŀ����
����Ŀ��2��13���ҹ��ϼ�����վ����̨��ɸĽ�����һ������վ����̨��Ϊ̽���ϼ���½�ؿǺ͵�ᣵ���ṹ��Ϊ�ҹ�ʵ��ȫ�����۲����罨���ṩ֧�š��������к��ƻ��������ת����ϵ���ش����⡣
��֪��B����ˮ��
��1��C�Ļ�ѧʽ��________,�����ճ������е���;_____________.
��2���������ij���ܸ��ӣ����й��о�����������бȽϻ�Ծ�������ж�����̼�ȡ�ͼ��A��C������������еؿ��Ҳ㷢������Ҫ��Ӧ֮һ���÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ___________����������е���ѹ������������̼�ڵ���ˮ�е��ܽ��Ҳ��________________��
��3��ʵ���ҳ���B������ȡ�����ռԭ��Ϊ______________���û�ѧ����ʽ��ʾ����
���𰸡�CaO ����ʳƷ����� CaCO3![]() CaO +CO2�� ���� Ca(OH)2+Na2CO3=2NaOH +CaCO3��
CaO +CO2�� ���� Ca(OH)2+Na2CO3=2NaOH +CaCO3��
��������
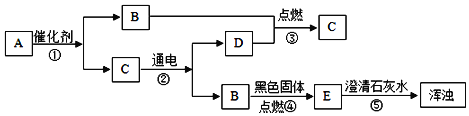
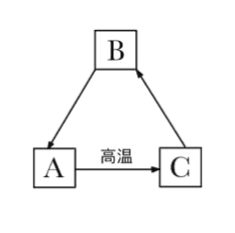
���ݿ�ͼ�ṩ����Ϣ����ϳ������ʼ��ת����̼����ܸ��·ֽ�Ϊ�����ƺͶ�����̼������������ˮ��Ӧ�����������ƣ������������������̼��Ӧ����̼��Ƴ�����ˮ����AΪ̼��ƣ�CΪ�����ƣ�BΪ�������ƣ������ͼ���ƶϺ�����
��1�����ݷ����Ʋ⣬CΪ�����ƣ���ѧʽΪCaO����Ϊ�����ƿ��Ժ�ˮ��Ӧ���ʿ�����ʳƷ�������
��2��AΪ̼��ƣ�CΪ�����ƣ�̼����ڸ��������·ֽ�Ϊ�����ƺͶ�����̼����ѧ����ʽΪCaCO3![]() CaO +CO2����
CaO +CO2����
��3��ѹ����������̼��ˮ�е��ܽ��Ҳ����
��4��BΪ�������ƣ��������ƺ�̼���Ʒ�Ӧ����̼��Ƴ������������ƣ���������ȡ�����ռ��Ӧ�ķ���ʽΪ��Ca(OH)2+Na2CO3=2NaOH +CaCO3����

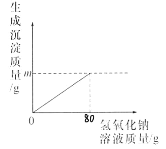
����Ŀ��Ϊ�ⶨij������Һ���������Ƶ������������ֱ�ȡ��Һ20g�������м��벻ͬ����������ͭ��Һ�������Һ�������ɷݾ���������ͭ��Һ��Ӧ����ʵ�����ݼ���������㣺
�������� ��Һ����/g | ����ͭ��Һ����/g | ���ɳ��� ����/g | |
��һ�� | 20 | 50 | 4.9 |
�ڶ��� | 20 | 100 | 9.8 |
������ | 20 | 150 | 9.8 |
��1��������������Һǡ����ȫ��Ӧʱ���ɳ���������Ϊ_____g��
��2���÷�Һ���������Ƶ���������_____��