��Ŀ����
 ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������
ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������
��1����Щ��ׯ���ȡ�õ���ˮ���������ˮ��Ӳˮ������ˮ�����õ�������________��
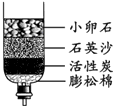
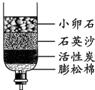
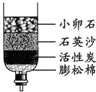
��2����Щ����ȡ���ǵĿ�ˮ��������ˮ����ͬѧ������ѧ��֪ʶ�����ǵĿ�ˮ��ͼ��ʾ�ļ���ˮ�����о���������С��ʯ��ʯӢɳ��������________��
��3���������ˮӲ�ȴ��߿�ˮ�в�ԭ������࣬�����Բ�ȡ________������������Ӳ�Ⱥ�ɱ��ԭ���
��4����������֮�����������Щʲô��________��________���о�����������
�⣺��1��Ӳˮ�����ˮ��ϲ��������ĸ�������ˮ�����ˮ��ϲ����϶����ĭ���������ˮ��Ӳˮ������ˮ�����õ������Ƿ���ˮ���������ˮ��
��2���ڼ���ˮ���У�С��ʯ��ʯӢɰ����ֹ�����Թ������ͨ�������˹��˵����ã�������ˣ�
��3��Ӳˮ�к��н϶�Ŀ����Ը�þ��������Ⱥ��ֽܷ����ɲ����Ը�þ��������ȿ���ɱ��ϸ��������������ˮӲ�ȴ��߿�ˮ�в�ԭ������࣬�����Բ�ȡ������з��������������У�
��4����������֮���������Ҫ��ƽʱ��������ע���Լ��ˮ�����Դ����ֹر�ˮ��ͷ������ˮ������ϴ��ˮ������ȷ������������������ֹر�ˮ��ͷ������ˮ������
�������������е�֪ʶ���з���������Ӳˮ����ˮʹ�õ��Ƿ���ˮ���ڼ���ˮ���У�С��ʯ��ʯӢɰ����ֹ�����Թ������ͨ�������˹��˵����ã�Ӳˮ�к��н϶�Ŀ����Ը�þ��������Ⱥ��ֽܷ����ɲ����Ը�þ��������ȿ���ɱ��ϸ�������ƽʱ��������Ҫע���Լ��ˮ��
���������⿼���˾�ˮ��֪ʶ����ɴ��⣬�����������е�֪ʶ�������������У�
��2���ڼ���ˮ���У�С��ʯ��ʯӢɰ����ֹ�����Թ������ͨ�������˹��˵����ã�������ˣ�
��3��Ӳˮ�к��н϶�Ŀ����Ը�þ��������Ⱥ��ֽܷ����ɲ����Ը�þ��������ȿ���ɱ��ϸ��������������ˮӲ�ȴ��߿�ˮ�в�ԭ������࣬�����Բ�ȡ������з��������������У�
��4����������֮���������Ҫ��ƽʱ��������ע���Լ��ˮ�����Դ����ֹر�ˮ��ͷ������ˮ������ϴ��ˮ������ȷ������������������ֹر�ˮ��ͷ������ˮ������
�������������е�֪ʶ���з���������Ӳˮ����ˮʹ�õ��Ƿ���ˮ���ڼ���ˮ���У�С��ʯ��ʯӢɰ����ֹ�����Թ������ͨ�������˹��˵����ã�Ӳˮ�к��н϶�Ŀ����Ը�þ��������Ⱥ��ֽܷ����ɲ����Ը�þ��������ȿ���ɱ��ϸ�������ƽʱ��������Ҫע���Լ��ˮ��
���������⿼���˾�ˮ��֪ʶ����ɴ��⣬�����������е�֪ʶ�������������У�

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ

 47��ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������
47��ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������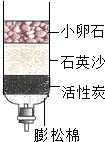 ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������
ȥ�궬�������괺�죬�ҹ����ϵ���������Χ�����ɺ���ȫ�������Ž�һ�£���ͬ������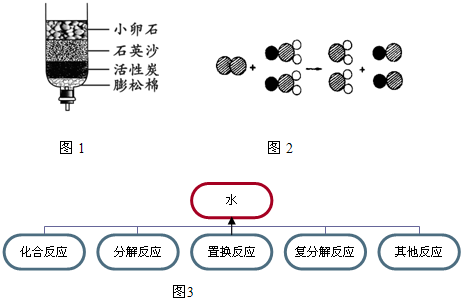
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�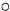 ��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ
��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ