��Ŀ����
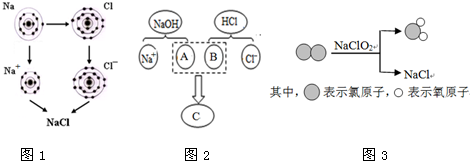
�Ȼ�������Ҫ�ĵ�ζƷ������������ȱ�ٵ�ζ������������ʾ��ͼ�ֱ��ʾ��ͬ�Ļ�ѧ��Ӧ�����������ж����Ȼ��ơ�
 |
ͼ1 ͼ2 ͼ3
��1��ͼ1�ǽ�������������Ӧ�����Ȼ��Ƶ���ʾ��ͼ����ͼ1��֪��Ԫ�صĻ�ѧ������ ������ĸ��ţ������еĹ�ϵ��
A. ���������� B. �ڲ������ C. ���Ӳ���
��2��ͼ2������NaOH��Һ�����ᷴӦ����ʵ�ʣ��÷�Ӧ�Ļ�����Ӧ����Ϊ ��ͼ��A��B��C��Ӧ����Ļ�ѧʽ�����ӷ�������Ϊ ��
��3��ͼ3��ʾ������������������������� ���ѧʽ�����÷�Ӧ�Ļ�ѧ����ʽΪ ��
��1��A ��2�����ֽ⣻ OH- H+ H2O ��3��ClO2 �� Cl2+2NaClO2 = 2NaCl + 2ClO2

��ϰ��ϵ�д�
�����Ŀ
 ��ѧ�����빫����ճ�����������أ�Ҳ�Dz��Ͽ�ѧ��������ѧ����Դ��ѧ���ִ���ѧ��������Ҫ�������Ȼ�������Ҫ�ĵ�ζƷ����ˮɹ�����Ȼ��Ƶ���Ҫ��Դ����ˮɹ��ͨ��������
��ѧ�����빫����ճ�����������أ�Ҳ�Dz��Ͽ�ѧ��������ѧ����Դ��ѧ���ִ���ѧ��������Ҫ�������Ȼ�������Ҫ�ĵ�ζƷ����ˮɹ�����Ȼ��Ƶ���Ҫ��Դ����ˮɹ��ͨ��������