��Ŀ����
����Ŀ��ij��ȤС��չ���˶�����ع��弰����Һ��ʵ��̽����������50��5%���������Һ��
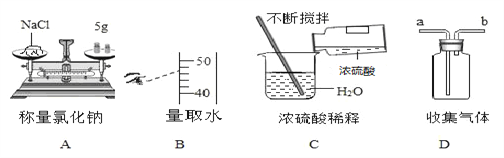
(1)������ȷ�IJ���˳����________________��
A.�ܢݢ٢ڢ� B.�٢ڢۢܢ� C.�ݢڢ٢ܢ� D.�ۢܢ٢ڢ�
(2)��ʵ�����ȡ����ع���_________g������ȡ����ʱ��������ƽ��ָ��������ƫ��ʱ������еIJ�����__________________________________________��
(3)������10g20%���������Һ(�ܶ�1.13g/cm3)����ϡ�ͳɸ�Ũ�ȵ���Һ������Ҫ����_____g��ˮ��ϡ�����г��ձ��Ͳ��������Ҫ��������__________________��
(4)��������Һ������ص���������ƫС������ԭ����______________��
A.��������ƽ��ȡʱ����߷������ұ߷������
B.ת������õ���Һʱ��������Һ�彦��
C.����Ͳȡˮʱ���Ӷ���
D.�ձ�������ˮ��ϴ����������Һ
���𰸡� A 2.5 ����������������������ع�����������ƽƽ�� 30 ��Ͳ�ͽ�ͷ�ι� ACD
��������(1). ����һ������������������Һ�IJ����Ǽ���������ܽ���װƿ��������Բ���˳���Ǣܢݢ٢ڢ� (2). 50��5%���������Һ������ص�����Ϊ��50��5%=2.5g�� (3). ����ȡ����ʱ��������ƽ��ָ��������ƫ��ʱ��˵�������Լ��������㣬����еIJ����Ǽ���������������������ع�����������ƽƽ�� (4). ��Һϡ���������ʵ�������������10g20%���������Һ��ϡ��5%����Һ������Ϊx��10g��20% =5%x,x=40�����Լ�ˮ������Ϊ40g-10g=30g��(5). ��ȡҺ���õ�����������Ͳ�ͽ�ͷ�ι� (6). ������Һʱ���ʵ���������ƫС��ԭ��������ƫ���������ܼ�ƫ����A.��������ƽ��ȡʱ����߷������ұ߷�����أ���������ƫ�٣�����Ͳȡˮʱ���Ӷ���������ƫС������ʵ��Һ��ƫ�ࣻ�ձ�������ˮ��ϴ����������Һ�������ܼ�ƫ�ࡣ

 ���ݼ���ϵ�д�
���ݼ���ϵ�д�����Ŀ����ͳ�ƣ��ҹ�ÿ�걨�ϵ��ֻ�����1�ڲ����������л������ã�����ɾ���˷Ѻ���Ⱦ��ͬѧ�ǶԷϾ��ֻ��еĽ������ղ�����Ȥ��
��������⡿ �ӷϾ��ֻ��п��Ի��յ���Щ�м�ֵ�Ľ�������λ��գ�
���������ϡ�
���ֻ���·���еĽ�������۸����±���ʾ��
���� | Fe | Cu | Al | Ni | Au | Ag | Pd���٣� |
�г��۸�/��$/t�� | 3 6 5 | 7175.5 | 1710.5 | 1 4 2 3 5 | 4.7��107 | 7.6��105 | 2 . 7 �� 1 0 7 |
��ʯ�к��� | 72.4 | 0.87 | 29.1 | 1.02 | 4 | 120 | 0.4 |
�ֻ���·���к��� | 5 | 13 | 1 | 0.1 | 350 | 1380 | 210 |
˵����Fe��Cu��Al��Ni�ĺ���������������%����ʾ��Au��Ag��Pd�ĺ����ĵ�λΪ��g/t����
��Ni�Ľ������λ����ǰ��Pd�Ľ������λ����� NiCl2��ҺΪ��ɫ��
��2Cu + O2 + 2H2SO4 ![]() 2CuSO4 + 2H2O
2CuSO4 + 2H2O
��ʵ�鷽����
��һ����ѡ��ֵ�û��յĽ���
�������Ϣ��е����ݣ��Ͼ��ֻ����л��ռ�ֵ�Ľ�����Au��Ag��Cu��Pd��ԭ���ǣ�
�� _____________�������ǵ��г��۸�ϸߡ�
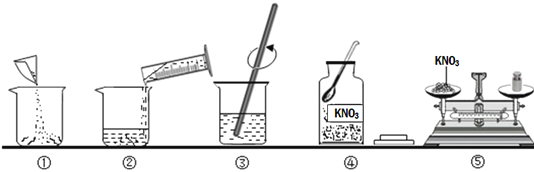
�ڶ��������ʵ����롢���ղ��ֽ���
ʵ�鲽�� | ʵ������ | |
I | ȡһ�Ͼ��ֻ���·�壬�õ紵���������·���ӵ�ĺ������ȷ磬һ��ʱ��������ӽ�������·�����ϰ���ק�� | �����ۻ� |
II | ��������·�����ձ��У��������ϡ������� | ���� |
III | ȡII��δ�ܽ�Ľ�����·��������ˮϴ��������ͼ��ʾװ���У�����ʵ�顣 | ��Һ�����ɫ����������������δ�ܽ� |
IV | ����III���ձ��е����ʣ�ϴ����ֽ�ϵ����� | ��ֽ���������������� |
V | ���� | ���� |
���������ۡ�
��1���ɲ���I�е�������֪�ĺ���������������____________��
��2������II��Ӧ��ʵ��������___________��
��3������II������Һ�У����ٺ���������_____________�֡�
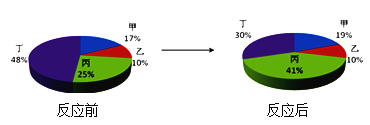
��4������V�IJ����ǣ�������������Һ�м���һ�������ۡ�������ʵ����̼�������ǰ����Һ����Ҫ���ӱ仯ʾ��ͼ����ͼ��ʾ��

�� ͼ�С�![]() �����������ķ���Ϊ____________��
�����������ķ���Ϊ____________��
�� ��ַ�Ӧ����ˣ���ֽ�Ϲ���������____________��д��ѧʽ����