��Ŀ����
��H��O��C��N��Ca��Na����Ԫ����ѡ���ʵ���Ԫ�ذ�Ҫ����գ�
��1�����ʵ������ֺͷ�����գ�
�ٶ���������________������������������________�������������-3�۵ĵ�Ԫ��________��
��2��д����������Ҫ������ʵĻ�ѧʽ��
�������������ʵĿ����к�������������________��
���������˹������������________
���������к����������ļ���________��
�ܾ������������Եķǽ���������________
��ˮ��Һ�ʼ��Ե�����________��
�⣺��1�����������ӣ�2Na+���������������ӣ�3OH--�����������-3�۵ĵ�Ԫ�أ�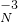 H4NO3��2���������������ʵĿ����к������������� N2�����������˹������������ CO2���������к����������ļ��� Ca��OH��2���ܾ������������Եķǽ���������C�� ��ˮ��Һ�ʼ��Ե�����Na2CO3��
H4NO3��2���������������ʵĿ����к������������� N2�����������˹������������ CO2���������к����������ļ��� Ca��OH��2���ܾ������������Եķǽ���������C�� ��ˮ��Һ�ʼ��Ե�����Na2CO3��
�ʴ�Ϊ����1��2Na+��3OH--��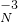 H4NO3��2��N2��CO2��Ca��OH��2��C��Na2CO3��
H4NO3��2��N2��CO2��Ca��OH��2��C��Na2CO3��
��������1�����������ӣ�2Na+���������������ӣ�3OH--�����������-3�۵ĵ�Ԫ�أ�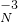 H4NO3
H4NO3
��2���������������ʵĿ����к������������� N2��
���������˹������������ CO2
���������к����������ļ��� Ca��OH��2��
�ܾ������������Եķǽ���������C��
��ˮ��Һ�ʼ��Ե�����Na2CO3��
���������⿼�黯ѧ�������д���京�壮�������������ʵĿ����к������������� N2��
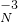 H4NO3��2���������������ʵĿ����к������������� N2�����������˹������������ CO2���������к����������ļ��� Ca��OH��2���ܾ������������Եķǽ���������C�� ��ˮ��Һ�ʼ��Ե�����Na2CO3��
H4NO3��2���������������ʵĿ����к������������� N2�����������˹������������ CO2���������к����������ļ��� Ca��OH��2���ܾ������������Եķǽ���������C�� ��ˮ��Һ�ʼ��Ե�����Na2CO3���ʴ�Ϊ����1��2Na+��3OH--��
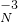 H4NO3��2��N2��CO2��Ca��OH��2��C��Na2CO3��
H4NO3��2��N2��CO2��Ca��OH��2��C��Na2CO3����������1�����������ӣ�2Na+���������������ӣ�3OH--�����������-3�۵ĵ�Ԫ�أ�
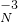 H4NO3
H4NO3��2���������������ʵĿ����к������������� N2��
���������˹������������ CO2
���������к����������ļ��� Ca��OH��2��
�ܾ������������Եķǽ���������C��
��ˮ��Һ�ʼ��Ե�����Na2CO3��
���������⿼�黯ѧ�������д���京�壮�������������ʵĿ����к������������� N2��

��ϰ��ϵ�д�
�����Ŀ