��Ŀ����
��10�Ĵ�����26����10�֣�ʵ���ҳ�������װ������ȡ������



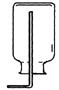
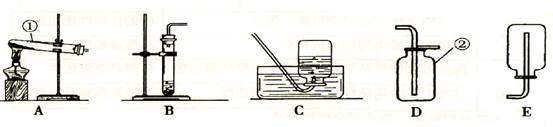
A B C D E
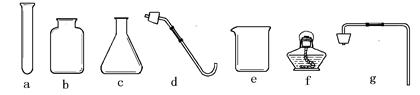
(1)д��ͼ���б�����������ƣ�a ��b ��
(2)��˫��ˮ�Ͷ�����������ȡ����ʱ����ѡ�õķ���װ���� ������ţ������ж��������� ���á��˷�Ӧ�Ļ�ѧ����ʽΪ ��
(3)ʵ���ҳ����Ȼ�粒�������ʯ�ҹ��干������ȡ������������NH3��һ����ɫ���д̼�����ζ�����壬�ܶȱȿ���С����������ˮ����ˮ��Һ���ԡ�
����ȡ������Ӧ�ķ���ʽΪ2NH4Cl +Ca(OH)2 CaCl2+2 X+ 2NH3����X�Ļ�ѧʽΪ ��
CaCl2+2 X+ 2NH3����X�Ļ�ѧʽΪ ��
����ȡ���ռ�NH3��Ӧ�ô���ͼ��ѡ��ķ���װ���� ���ռ�װ���� ��
��NH3��һ�ּ������壬����ʱ����ѡ�����и�����е� ������ţ���
A.������������ B.Ũ���� C.��ʯ��





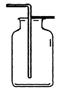
A B C D E
(1)д��ͼ���б�����������ƣ�a ��b ��
(2)��˫��ˮ�Ͷ�����������ȡ����ʱ����ѡ�õķ���װ���� ������ţ������ж��������� ���á��˷�Ӧ�Ļ�ѧ����ʽΪ ��
(3)ʵ���ҳ����Ȼ�粒�������ʯ�ҹ��干������ȡ������������NH3��һ����ɫ���д̼�����ζ�����壬�ܶȱȿ���С����������ˮ����ˮ��Һ���ԡ�
����ȡ������Ӧ�ķ���ʽΪ2NH4Cl +Ca(OH)2
 CaCl2+2 X+ 2NH3����X�Ļ�ѧʽΪ ��
CaCl2+2 X+ 2NH3����X�Ļ�ѧʽΪ ������ȡ���ռ�NH3��Ӧ�ô���ͼ��ѡ��ķ���װ���� ���ռ�װ���� ��
��NH3��һ�ּ������壬����ʱ����ѡ�����и�����е� ������ţ���
A.������������ B.Ũ���� C.��ʯ��
��10�֣�(1) �Թ� ����̨(2) B �� 2H2O2  2H2O+O2��
2H2O+O2��
(3) �� H2O �� A �� B
 2H2O+O2��
2H2O+O2��(3) �� H2O �� A �� B
����1����ͼ�пɿ�����a�Ǽ����������оƾ��ƣ�b�ǹ̶��Թܵ�������������̨��
��2��˫��ˮ���������⣬��Һ�壬���������ǹ��壬������ȼ��ɲ������������ڹ̹̳����ͣ���ѡ��Bװ�ã����ж�������������ã�˫��ˮ�Ͷ�����������ȡ���������û�ѧ����ʽ��ʾΪ��2H 2O 2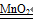 2H 2O+O 2����
2H 2O+O 2����
��3����Ҫȷ��X�Ļ�ѧʽ�������������غ㶨�ɣ��ڻ�ѧ��Ӧǰ��ԭ�ӵ��������Ŀ�����䣬�����Ƴ�X�Ļ�ѧʽΪH2O��
��������֪ʵ�����ư��������Ȼ�粒������ʯ�ҹ��干������ȡ�ģ����ù̹̼�����װ�ã���ѡA��������������ˮ�����Բ�������ˮ���ռ�����������Ϊ�����ܶȱȿ���С�ö࣬���������ſ������ռ���������ѡ��D��
��������֪�����ʼ��ԣ����ü��Եĸ������������������Եĸ���������Ϊ���ᷢ���кͷ�Ӧ��
A�������������ƣ��Ǽ���Ը��ﰱ������A�������⣻
B��Ũ���ᣬ���ᣬ�ܺͰ���������Ӧ�����Ũ��������ڸ��ﰱ������B�������⣻
C����ʯ�ң��������Ƶ��׳ƣ��백��Ҳ����Ӧ�������ڸ��ﰱ������C�������⣻
�ʴ�ѡB��
�ʴ��ǣ�
��1���ƾ��ơ�����̨��
��2��B������ 2H 2O 2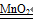 2H 2O+O 2����
2H 2O+O 2����
��3����H2O����A��D����B��
��2��˫��ˮ���������⣬��Һ�壬���������ǹ��壬������ȼ��ɲ������������ڹ̹̳����ͣ���ѡ��Bװ�ã����ж�������������ã�˫��ˮ�Ͷ�����������ȡ���������û�ѧ����ʽ��ʾΪ��2H 2O 2
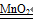 2H 2O+O 2����
2H 2O+O 2������3����Ҫȷ��X�Ļ�ѧʽ�������������غ㶨�ɣ��ڻ�ѧ��Ӧǰ��ԭ�ӵ��������Ŀ�����䣬�����Ƴ�X�Ļ�ѧʽΪH2O��
��������֪ʵ�����ư��������Ȼ�粒������ʯ�ҹ��干������ȡ�ģ����ù̹̼�����װ�ã���ѡA��������������ˮ�����Բ�������ˮ���ռ�����������Ϊ�����ܶȱȿ���С�ö࣬���������ſ������ռ���������ѡ��D��
��������֪�����ʼ��ԣ����ü��Եĸ������������������Եĸ���������Ϊ���ᷢ���кͷ�Ӧ��
A�������������ƣ��Ǽ���Ը��ﰱ������A�������⣻
B��Ũ���ᣬ���ᣬ�ܺͰ���������Ӧ�����Ũ��������ڸ��ﰱ������B�������⣻
C����ʯ�ң��������Ƶ��׳ƣ��백��Ҳ����Ӧ�������ڸ��ﰱ������C�������⣻
�ʴ�ѡB��
�ʴ��ǣ�
��1���ƾ��ơ�����̨��
��2��B������ 2H 2O 2
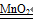 2H 2O+O 2����
2H 2O+O 2������3����H2O����A��D����B��

��ϰ��ϵ�д�
�����Ŀ