��Ŀ����
��ѧ�����ǵ����������������أ�ѧ�û�ѧ֪ʶ��ʹ���Ǹ��õ���ʶ�ͽ��ʵ�����⣮��1�����dz���ϴ�Ӽ���ϴ�;��ϵ����ۣ�������Ϊϴ�Ӽ����� �Ĺ��ܣ�
��2�����õļҾӻ����������õ����
�ٷ���װ�������ڷ�һЩ����̿������װ�����ͷų��ļ�ȩ�������ж����壬�������û���̿�� �ԣ�
����ͼ��ʾ�ġ�����Ϩ����һ�����͵ļ��������Ʒ��������Ϩ���Ӵ�������3-5���ը�����ͷŵķ�ĩ������ȼ�����ϣ�ͬʱ�ų�����ȼ�����壬ʹ����Ϩ�𣮡�����Ϩ�������ԭ���� �������ţ�
A�������ȼ��
B��ʹȼ��������������
C������ȼ������Ż��
��3���������ִ������г�����һ�ֽ�ͨ���ߣ�
����������ӻ�����������ǵ���ʴ�������ԭ���Ǹ��� ��
��CNG˫ȼ�ϻ����������ڸ���Ͷ��ʹ�ã������������õ�ȼ�������ͺ�ѹ����Ȼ����д����Ȼ����ȫȼ�յĻ�ѧ����ʽ�� ��
��4��ʳ������Ҫ�ĵ�ζƷ�������г���Ϊ�������ʶ�����ֱ��ʳ�ã����dz����Ѵ���ͨ�� �� �� ���������ᴿΪ���Σ�

���𰸡��������������е�֪ʶ���з�����ϴ�ྫ�����黯���ã�����̿���������ԣ�������ɫ�غ���ζ���������ƻ�ȼ�յ�������������ˮ����������ʱ�����⣬�����е����ʿ���ͨ���ܽ⡢���ˡ������ķ�����ȥ���ݴ˽�ɣ�
����⣺��1��ϴ�ྫ�����黯���ܣ���������ϴ���ۣ�����黯��
��2���ٻ���̿���������ԣ�����������ɫ�غ���ζ�����������
���ͷŵķ�ĩ������ȼ�����ϣ�ͬʱ�ų�����ȼ�����壬�����ǿ�ȼ���������������ﵽ����Ŀ�ģ����B��
��3��������������������ʹ����ˮ�������������Ӷ���ֹ���⣬���������ˮ��
�ڼ���ȼ�պ����ɶ�����̼��ˮ�����CH4+2O2 CO2+2H2O��
CO2+2H2O��
��4�������е����ʿ���ͨ���ܽ⡢���ˡ������ķ�����ȥ������ܽ⣬���ˣ�����
���������⿼���˻�ѧ��������й�֪ʶ����ɴ��⣬�����������еĿα�֪ʶ�������ṩ����Ϣ���У�Ҫ��ͬѧ�Ǽ�ǿ�Ի���֪ʶ�Ĵ������Ա����Ӧ�ã�
����⣺��1��ϴ�ྫ�����黯���ܣ���������ϴ���ۣ�����黯��
��2���ٻ���̿���������ԣ�����������ɫ�غ���ζ�����������
���ͷŵķ�ĩ������ȼ�����ϣ�ͬʱ�ų�����ȼ�����壬�����ǿ�ȼ���������������ﵽ����Ŀ�ģ����B��
��3��������������������ʹ����ˮ�������������Ӷ���ֹ���⣬���������ˮ��
�ڼ���ȼ�պ����ɶ�����̼��ˮ�����CH4+2O2
 CO2+2H2O��
CO2+2H2O����4�������е����ʿ���ͨ���ܽ⡢���ˡ������ķ�����ȥ������ܽ⣬���ˣ�����
���������⿼���˻�ѧ��������й�֪ʶ����ɴ��⣬�����������еĿα�֪ʶ�������ṩ����Ϣ���У�Ҫ��ͬѧ�Ǽ�ǿ�Ի���֪ʶ�Ĵ������Ա����Ӧ�ã�

��ϰ��ϵ�д�
�����Ŀ
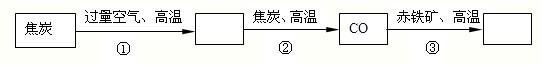

 11����ѧԴ�����ͬʱ�ַ���������ճ������������ѧ�������ǵ������벻����ѧ���ش��������⣮
11����ѧԴ�����ͬʱ�ַ���������ճ������������ѧ�������ǵ������벻����ѧ���ش��������⣮
