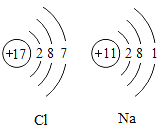
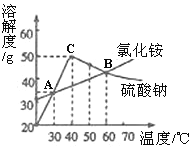
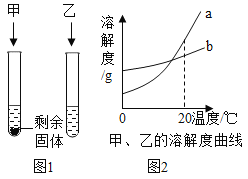
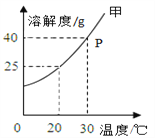
��Ŀ����
����Ŀ��ʵ�����ʱ��ͬѧ�ǽ�����CuSO4��ZnSO4��FeSO4�ķ�Һ���ڷ�Һ����������Һֱ���ŷžͻ����ˮ��Ⱦ�����Ǽ�λͬѧ���ÿ��ദ����Һ�����չ�ҵ��Ҫԭ������п���йؽ�����ʵ���������:
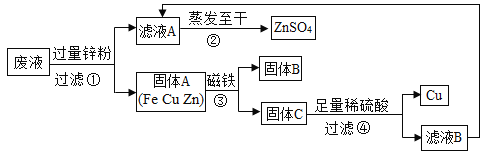
��1����ҺA����ҺB������ͬ�����ʣ���������_____;����B�Ļ�ѧʽΪ______.
��2��д�����������һ����Ӧ�Ļ�ѧ����ʽ______������ܷ�����Ӧ�Ļ�ѧ����ʽ_______��
��3��Ҫ���鲽����м����ϡ�����Ƿ������ķ�����________��
��4����ʵ������е�������ʧ���Ժ��ԣ�Ҫ����÷�Һ������п�������������������:��Һ��������____������
���𰸡�����п �� Zn+ CuSO4 == ZnSO4 + Cu��(��Zn+FeSO4= ZnSO4 +Fe) Zn+ H2SO4 == ZnSO4 + H2�� ȡ����ܵ��������Թ��м�������ϡ���ᣬ�������ݲ���������� ����п��������п�۵�����
��������
��1��������CuSO4��ZnSO4��FeSO4�ķ�Һ���չ�ҵ��Ҫԭ������п���йؽ�������ҺA����ҺB������ͬ�����ʣ������ᾧ�õ��ľ���������п������Һ�е�����������п�����ܱ���������������B��������ѧʽΪFe��
��2��п�Ļ�Ա�����ͭ�Ļ��ǿ��п��������ͭ������������Ӧ������ٷ�Ӧ�Ļ�ѧ����ʽZn+ CuSO4 == ZnSO4 + Cu�� Zn+FeSO4= ZnSO4 +Fe������ܷ�����Ӧ��п�����ᷴӦ��������п����������ѧ����ʽΪZn+ H2SO4 == ZnSO4 + H2����
��3��������м���������ϡ�����Ŀ���ǽ�п��ȫ��Ӧ������������������������ù�����ֻ��ͭ�������㣬�����ù�������ͭ��п��Ҫ���鲽����м����ϡ�����Ƿ������ķ����ǽ���������ù����м������ᣬ������������壬˵����������
��4���������ʵ���������=![]() ��100%��֪Ҫ����÷�Һ������п�����������������������Һ������������п��������п�۵�������
��100%��֪Ҫ����÷�Һ������п�����������������������Һ������������п��������п�۵�������

 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�