��Ŀ����
 ��ѧ�����������ߣ��������ǵ�����ϢϢ��أ�
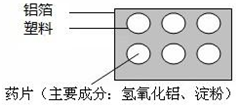
��ѧ�����������ߣ��������ǵ�����ϢϢ��أ���1����ͼΪij����ҩ��ʵ��ͼ����ش��������⣺
��ͼ�б�ʾ�������У����еĽ���Ԫ���ǣ���Ԫ�ط��ţ�
��ҩƬʹ�����Ժ����ϰ�װ��������ʵ�飬�����൱�ڵ�ΰ壬���ŵ���
��2������H��C��O��N���ֳ�����Ԫ�أ���ѡ�����е�Ԫ��д����������Ҫ������ʸ�һ�֣��û�ѧʽ��ʾ����
����֧��ȼ�յ�����
�ۿ����к�����������
��������1���ٸ���ij����ҩ��ʵ��ͼ���ҳ�����Ԫ�أ�д��Ԫ�ط��ţ�д����Ҫ�ɷ����������Ļ�ѧʽ��
�ڸ��ݵ�ΰ���ص�����ŵ㣬����п�����ᷴӦ�����ʡ�д����Ӧ�ķ���ʽ��
��2���������ʵ����ʡ����Ԫ�أ�ѡ��Ԫ��������ʣ�д����ѧʽ��
�ڸ��ݵ�ΰ���ص�����ŵ㣬����п�����ᷴӦ�����ʡ�д����Ӧ�ķ���ʽ��
��2���������ʵ����ʡ����Ԫ�أ�ѡ��Ԫ��������ʣ�д����ѧʽ��
����⣺��1�����ɿ���ҩ��ʵ��ͼ��֪�����ڽ���Ԫ�ص�������Ԫ�ط���Ϊ��Al����ҩƬ��Ҫ�ɷ����������Ļ�ѧʽ�ǣ�Al��OH��3��
�ڸ����ϰ�װ��������ʵ�飬�����൱�ڵ�ΰ壬���ŵ��ǣ���ԼҩƷ�����ø����ϰ�װ����ʵ��ܶ࣬���磬п������ķ�Ӧ����ѧ����ʽΪ��Zn+H2SO4=ZnSO4+H2����
��2������֧��ȼ�յ���������������ѧʽ�ǣ�O2����Ǧ��о�е���Ҫ������ʯī����ѧʽ�ǣ�C��
�ۿ����к������������ǵ�������ѧʽ�ǣ�N2���ܿ������˹�����������Ǹɱ�����ѧʽ�ǣ�CO2��
�ʴ�Ϊ����1����Al�� �ڽ�ԼҩƷ��Zn+H2SO4=ZnSO4+H2������2����O2����C����N2����CO2��
�ڸ����ϰ�װ��������ʵ�飬�����൱�ڵ�ΰ壬���ŵ��ǣ���ԼҩƷ�����ø����ϰ�װ����ʵ��ܶ࣬���磬п������ķ�Ӧ����ѧ����ʽΪ��Zn+H2SO4=ZnSO4+H2����
��2������֧��ȼ�յ���������������ѧʽ�ǣ�O2����Ǧ��о�е���Ҫ������ʯī����ѧʽ�ǣ�C��
�ۿ����к������������ǵ�������ѧʽ�ǣ�N2���ܿ������˹�����������Ǹɱ�����ѧʽ�ǣ�CO2��
�ʴ�Ϊ����1����Al�� �ڽ�ԼҩƷ��Zn+H2SO4=ZnSO4+H2������2����O2����C����N2����CO2��
����������ͨ������ҩ��ʵ��ͼ�������������еĻ�ѧ֪ʶ�������˻�ѧ�����������ߣ�ѧ�û�ѧ֪ʶ������������е�һЩ���⣮

��ϰ��ϵ�д�
�����Ŀ
