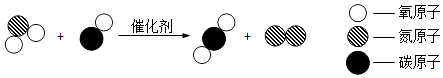��Ŀ����
����Ŀ��̼��ѧһֱ�ǿ�ѧ���о�����Ҫ���⡣
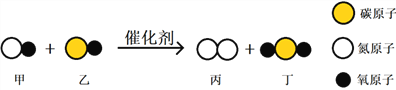
(1)�������ɸ����е�������������ҪĿ����__________________��
(2)���쵽�ˣ��������в��ټ�ͥ����ȼúȡů��ȼúʱ����С�ĵĻ����������һ����̼�ж�����ԭ����ú(��Ҫ�ɷ���̼)�IJ���ȫȼ������ģ����û�ѧ����ʽ��ʾ��һ����___________�����ȼúʱһ��Ҫע�����ڵ�ͨ�绻����С��ͬѧ��Ϊ���������ڷż���ˮ����ֹһ����̼�ж�������Ϊ������________��������_____________________��
(3)12��11�գ���������ʩͶ������(�������Ͷ����)�����������Ѿ���һ��2.5����Ԫ�Ĵ�����ڱ�����ú�������̡�������ѧ����֪ʶ������Ϊú����������ŵ���______________��
(4)��¯β���к��д�����һ����̼���壬������Ի������á���д��һ����̼��һ����;��________��
(5)��ѧ��ȤС���ͬѧ���ijһ��̼������(CxHy��CxHyOz )����ɽ���̽������С��ͬѧ���Ȳ���˸û��������Է�������Ϊ46��Ȼ���ֽ�4.6g�û�������ȫȼ�գ��õ���8.8gCO2��5.4gH2O��ͨ��̽������ȤС���ͬѧ�õ��Ļ�����Ļ�ѧʽΪ________���û�������ȫȼ�յĻ�ѧ����ʽΪ______________________________��
���𰸡� ���ͺ�̼�� 2C+ O2![]() 2CO ���� һ����̼������ˮ ������Ⱦ ��ȼ�� C2H6O C2H6O+3O2
2CO ���� һ����̼������ˮ ������Ⱦ ��ȼ�� C2H6O C2H6O+3O2![]() 2CO2+3H2O
2CO2+3H2O
����������1�� �������ɸ����е����������ǽ�����ת��Ϊ�֣�����ҪĿ���ǽ��ͺ�̼����
��2��ú����Ҫ�ɷ���̼���IJ���ȫȼ������һ����̼����ѧ����ʽΪ��C+ O2![]() CO������һ����̼������ˮ�����ڷż���ˮ���ܷ�ֹһ����̼�ж���
CO������һ����̼������ˮ�����ڷż���ˮ���ܷ�ֹһ����̼�ж���
��3����Ȼ�������ú��ȼ�������ɵĿ�����Ⱦ����٣�����ú����������Ⱦ��
��4��һ����̼���п�ȼ�ԣ�������ȼ�ϣ����л�ԭ�ԣ�����ұ��������
��5��8.8g������̼��̼Ԫ�ص�����Ϊ��8.8g��![]() ��100%=2.4g��5.4gˮ����Ԫ�ص�����Ϊ��5.4g��
��100%=2.4g��5.4gˮ����Ԫ�ص�����Ϊ��5.4g��![]() ��100%=0.6g������������Ԫ�ص�����Ϊ��4.6g-2.4g-0.6g=1.6g��
��100%=0.6g������������Ԫ�ص�����Ϊ��4.6g-2.4g-0.6g=1.6g��
�������У�̼Ԫ�ء���Ԫ�غ���Ԫ�ص������ֱ���2.4g��0.6g��1.6g��̼ԭ�ӡ���ԭ�Ӻ���ԭ�ӵĸ�����Ϊ�� ![]() =2��6��1���������ʵĻ�ѧʽ��C2H6O����ȫȼ�յĻ�ѧ����ʽΪ��C2H6O+3 O2
=2��6��1���������ʵĻ�ѧʽ��C2H6O����ȫȼ�յĻ�ѧ����ʽΪ��C2H6O+3 O2![]() 2CO2+ 3H2O��
2CO2+ 3H2O��

����Ŀ�����й��ڻ�ѧʵ��ġ�Ŀ�ġ������������������ۡ���������ȷ���ǣ� ��
ѡ�� | ʵ��Ŀ�� | ʵ����� | ʵ������ | ʵ����� |
A | ����ϡ���������������Һ�Ƿ�ǡ����ȫ��Ӧ | �ڷ�Ӧ�����Һ�еμ���ɫ��̪��Һ | ���������� | ǡ����ȫ��Ӧ |
B | ����ij�����Ƿ���CO2���� | ������ͨ����ɫʯ����Һ�� | ��Һ��� | ԭ����һ����CO2 |
C | ����ˮ������ˮ | �����ᾧ | Һ����ʧ�������� | ��Һ��Ϊ����ˮ |
D | ���װ�õ������� |
| ����Һ����ƽ | ���������� |
A. A B. B C. C D. D