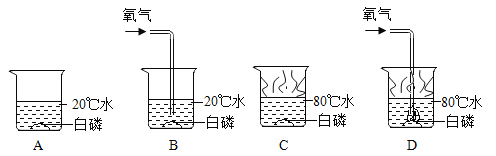
��Ŀ����
����Ŀ����2018ʯ��ׯ�������ʼ죩ij�о�С�鷢�֣�άC����Ƭ������ҩƷ����Ҫ�ɷּ�ͼ1������ˮ�����������ݲ�������ͼ2������С��ͬѧ���������о���
̽��һ��������ijɷ�
����������裩С��˵�������������CO2��O2��CO��H2��N2��
С��˵�������ܺ���CO��H2����Ϊ��ҩƷ��ȫ�Ƕȿ��ǣ�CO�ж���H2________________��
С��˵�������ܺ���N2����Ϊ___________��
��С��ͬѧ��Ϊ����������ܺ���CO2��O2�е�һ�ֻ����֡�
������ʵ�飩
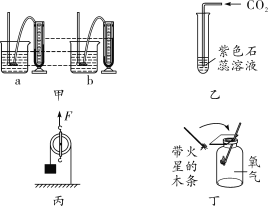
ʵ���� | ʵ����� | ʵ������ |
�� | ������ͨ�����ʯ��ˮ�� | ����ʯ��ˮ����� |
�� | �������ǵ�ľ������������� | �����ǵ�ľ��û�и�ȼ |
���ó����ۣ�
��1����ʵ��ٿ�֪���������п϶�����________��д���÷�Ӧ�Ļ�ѧ����ʽ________��
��2����ʵ��ڲ���ȷ���������в���������������_____________��
��Ҫ֤����������������������ʵ��_____________��
̽������άC����Ƭ��Һ�������
��3����άC����Ƭ��Һ�еμ�ʯ����Һ����Һ��죬˵����Һ��________�ԡ�
���𰸡���ȼ�ױ� ��Ӧ���в����е�Ԫ�� CO2���������̼�� Ca��OH��2��CO2=CaCO3����H2O ����������������������Сʱ������ʹ�����ǵ�ľ����ȼ ��������ͨ������������������Һ�г�ȥ������̼�������ʣ�������ô����ǵ�ľ������ ��
��������
̽��һ������������衿�����ܺ���H2����Ϊ��ҩƷ��ȫ�Ƕȿ��ǣ�H2��ȼ�ױ��������ܺ���N2����Ϊ��Ӧ���в����е�Ԫ�ء����ó����ۡ���1����ʵ��ٿ�֪����ʹ����ʯ��ˮ����ǣ��������п϶����ж�����̼����Ӧ�Ļ�ѧ����ʽΪCa��OH��2��CO2=== CaCO3����H2O����2����ʵ��ڲ���ȷ���������в�������������Ϊ����������������������Сʱ������ʹ�����ǵ�ľ����ȼ����Ҫ֤�������������ɽ�������ͨ����������������Һ�г�ȥ������̼�������ʣ�������ô����ǵ�ľ�����飬��ľ����ȼ����ԭ�����к���������̽��������3����άC����Ƭ��Һ�еμ�ʯ����Һ����Һ��죬˵����Һ�����ԡ�

����Ŀ��������ij��ѧ��ѧ��ȤС���ͬѧ�����й�����ֽ�����տ���ͬѧ�ǿ�����Ʒ��ǩ�ϱ�����ֽ�IJ���Ϊ����������
��������⣩��ֽ�IJ��ʵ��������������أ�С����Ϊ������ֽ���������й۲��Ƿ�������ų�������������������С��ͬѧ��Ϊ������������������___________��
Сʩ�������Ϸ��������۵���232 �����������۵�Ϊ660 ��������_______�ķ����������ֽ�IJ�������������������ͬѧ�Ƕ������ֽ���������̽����
��̽���һ�����ĺ�������
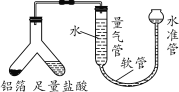
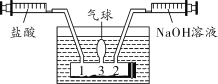
��1��Сʩ����ͼװ�òⶨ�����н������ĺ����������װ�������ԵIJ�����___________��
��2������ý��������ܵ��������Ϊ100 mL���������ܶ�Ϊ0.09 g/L���������н�����������Ϊ______ g��������ֻ�����������ᷴӦ�������壩��
��̽�����������ȼ��
ͬѧ��������ȼ�յ�ʵ�顣С��������ǯ�г�һС���������ھƾ��ƻ����ϣ�����ζ����۲쵽�����ۻ���ʧȥ�˹�����������ȼ�գ�С����ȡһ����������ɰֽ��ϸ��ĥ����������ǯ�гַ��ھƾ��ƻ����ϣ��۲쵽�����Բ���ȼ�ա�
��1��Сʩͬѧ��Ϊ���������ڿ�����ȼ������Ϊ__________��
��2��Сʩͬѧ�Ľ���ʵ�飬������ȼ�ղ�������ҫ�۵İ⣬Сʩͬѧ��ʵ�鷽����_________��
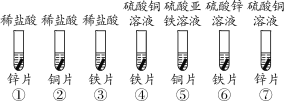
��̽�������С�⽫��ĥ�����Ƭ��ͭ����Һ��Ӧ���������£�
ʵ��һ��4 mL 8%��CuSO4��Һ | ʵ�����4 mL 8%��CuCl2��Һ | |
ʵ�� ���� | ��ʱ�����ޱ仯��һ��ʱ�����Ƭ�ϲų���������ɫ��ͭ | ��Ƭ��Ѹ���к�ɫ��ͭ���� |
��1�������Ȼ�ͭ��Һ��Ӧ�Ļ�ѧ����ʽΪ__________��
��2���Ա�����ʵ�飬С���Ʋ������ͭ����Һ�ķ�Ӧ�����дٽ����õ�������__________����д���ӷ��ţ���Ϊ����֤���Ʋ⣬Сʩ��ʵ��һ��CuSO4��Һ�м���1g________����д��ѧʽ�����壬�����۲쵽��Ƭ�ϳ��ִ�����ɫ��ͭ��