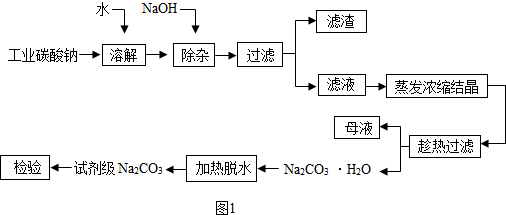
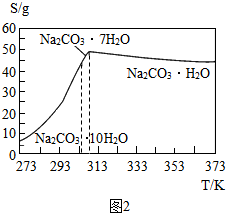
��Ŀ����
 ��ˮ���ֱ�����Դ��
��ˮ���ֱ�����Դ���١���ˮɹ�Ρ������õķ�����
I����ȴ�ᾧ II�������ᾧ III������
�ں��Ϻ���ȱ������ˮʱ�ɲ�����ͼװ�û�õ�ˮ���ø�װ�ý�2000g���Ȼ���3%�ĺ�ˮ��ɹ4Сʱ��ʣ�ຣˮ���Ȼ��Ƶ���������Ϊ4%�����ռ����Ŀ�����ˮΪ
�ۺ�ˮ�е����ᣨ��ѧʽΪH3BO3�������ڲ�����ҵ���±�Ϊ����IJ����ܽ�����ݣ�
| �¶ȣ��棩 | 0 | 20 | 40 | 60 | 80 |
| �ܽ�ȣ�g/100gˮ�� | 3 | 5 | 9 | 15 | 23 |
��60��ʱ��100g���ᱥ����Һ�к�������
��ijͬѧ�������ʵ��̽�����¶ȡ������������ʵ���̬�����ܽ����ʵ�Ӱ�죮
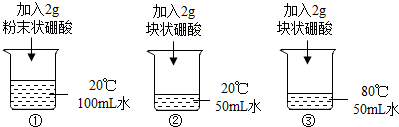
a�����������ձ��У�������ҺŨ�ȵĴ�С��ϵ��
A����20���ˮ��Ϊ80��B����ˮ�������Ϊ50mL C������ĩ״�����Ϊ��״��
��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
�����Ŀ
��ͼ��ʾ�ı�־�����ڽ�ֹ���̵��ǣ�������
A�� | B�� | C�� | D�� |
 �����ܼ��ţ���̼���������ּ�ڳ�����Լ��Դ�����������Դ�������������������̼���ŷţ����д�ʩ�в����ϸ�������ǣ�������
�����ܼ��ţ���̼���������ּ�ڳ�����Լ��Դ�����������Դ�������������������̼���ŷţ����д�ʩ�в����ϸ�������ǣ�������| A���㷺ʹ��̫���ܺͷ��ܵ������Դ | B���������÷Ͼɽ��� | C����ʹ��һ����������Ʒ | D������ʹ��˽�ҳ���������˹������������г� |
���й�����Դ��������ȷ���ǣ�������
| A����嫵ĺ����к��зḻ����Դ�����к��������������Ȼ��� | B����ú����������ǿ�ȿɵõ���¯ú����ú���ͺ�Һ��ʯ�����ȶ��ֲ�Ʒ | C���ؿ��еĽ���Ԫ���ǹ�Ԫ�أ�������Ӧ����㷺��Ҳ����Ԫ�� | D���õ������ʳƷ��װ������������ |
��ˮ�����ӵķֲ������ͼ��ʾ���ɴ˵õ��Ľ�����ȷ���ǣ�������


| A����ˮ�����Ӵ�������ɶฺ����� | B����ˮ����Һ����һ�ȶ���ԭ�������ܽ�����Ӵ�С��ˮ���Ӳ�� | C���������ɫ����������ˮ������õ�����һ������ɫ�Ĺ��� | D����ˮ�ijɷ��ǹ̶������ |
�����������Ƽ���Ŀ�ѧ���ǣ�������
| A����°� | B���Ž��з� | C�������� | D�������� |
��NaCl+CO2+NH3+H2O�TNaHCO3+NH4Cl���������ġ������Ƽ������Ҫ��Ӧ����Ӧ���ɵ�NaHCO3��һ���Σ��ﵽһ��Ũ��ʱ�����Һ���Ƚᾧ������������4λͬѧ�Ը÷�Ӧ�漰���й�֪ʶ�����IJ��ּ��⣬������ȷ���ǣ�������
| A�������Ӧ���ڸ��ֽⷴӦ | B��NaHCO3һ����������ˮ������ | C����Ӧ�����Һ�д���NH4+ | D���ᾧ��NaHCO3�����Һ��û��Na+ |
���н�������Ա���������Դû�����õ��ǣ�������
| A����������Ѱ�ҽ�������Ʒ�Ŀ����о� | B����ǿ�Ͼɽ����Ļ��պ������� | C����ɽ������ | D����ֹ������ʴ |