��Ŀ����
̼�������������һ��Ԫ�أ�̼�Ļ���������Ȼ������Ҫ�����ʣ���1����Ԫ�����ڱ��У�̼Ԫ�ص���Ϣ��ͼ��ʾ��̼Ԫ�ص����ԭ������Ϊ ��
��2�������CO2�ŷŻ��������ЧӦ��Ϊ�˼���������CO2���������ӣ�Ŀǰ���½�����е��� ������ĸ��ţ���
A�����ˮ�������ȼ������
B������ȼú�¼����������ȼ��
C����ֹʹ��ú��ʯ�͡���Ȼ����ȼ��
D������̫���ܡ����ܡ������ܵ�����Դ
��3���״���CH3OH����һ����������ȼ�ϣ�������ɫ���Դ��ƾ�ζ��Һ�壬��ȼ���ж����ܶȱ�ˮС������ˮ����ܣ��������ڼ״��������ʵ��� ��
��4����������Ŵ����ġ���ȼ����������Ϊδ��������Դ������Ҫ�ɷ��Ǽ���ˮ�������ȼ�յĻ�ѧ����ʽΪ ��
��5��ʯ��ʯ��;�dz��㷺�����������ջ������糧úȼ��ʱ�����Ķ�������������뽫��Ӧ�Ļ�ѧ����ʽ����������2CaCO3+2SO2+O2
 2CaSO4+ ��
2CaSO4+ ��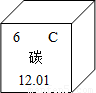
���𰸡���������1������Ԫ�����ڱ��ṩ����Ϣ����֪��Ԫ�ط��š�Ԫ�����Ƽ����ԭ���������н��
��2�����ݼ���������CO2�����Ĵ�ʩ���н��
��3���������ʵ���ɫ��״̬���ܶȡ�ˮ���Եȷ�������ʲ���Ҫͨ����ѧ�仯���ֳ����������������ʽ��н��
��4�����ݼ���ȼ�����ɶ�����̼��ˮ���н��
��5�����ݻ�ѧ��Ӧǰ��ԭ�ӵ��������Ŀ������н��
����⣺��1������Ԫ�����ڱ��ṩ����Ϣ����֪̼Ԫ�ص����ԭ������Ϊ12.01��
��2��A��������Ȼ�������Դ�������ˮ�������ȼ���������Dz���ʵ�ģ�Ŀǰ�����У�
B��ʹú���ȼ�գ�Ҳͬ�������ɴ����Ķ�����̼���������������CO2������
C��ú��ʯ�͡���Ȼ���ȿ���ȼ�����ִ�������Ҫԭ�ϣ����ܽ�ֹʹ�ã��˷�Ŀǰ����ʵ��
D����������Դ�����ٶ�����̼���ŷţ����Լ�������ЧӦ������Ŀǰ���У�
��3������Ҫͨ����ѧ�仯���ֳ��������ʾ����������ʣ��������ڼ״��������ʵ��ǣ���ɫ���Դ��ƾ�ζ��Һ�塢�ܶȱ�ˮС������ˮ����ܣ�
��4������ȼ�����ɶ�����̼��ˮ����ѧ����ʽΪCH4+2O2 CO2+2H2O��
CO2+2H2O��
��5���ɷ�Ӧ�Ļ�ѧ����ʽ2CaCO3+O2+2SO2�T2CaSO4+X�����ݻ�ѧ�仯ǰ��ԭ�ӵ����ࡢ��Ŀ���䣬���ж�������X�ĺ���2��Cԭ�Ӻ�4��Oԭ�ӣ���ÿ��������1��Cԭ�Ӻ�2��Oԭ�ӹ��ɣ����ʵĻ�ѧʽΪCO2��
�ʴ�Ϊ����1��12.01����2��D����3����ɫ���Դ��ƾ�ζ��Һ�塢�ܶȱ�ˮС������ˮ����ܣ���4��CH4+2O2 CO2+2H2O����5��2CO2��
CO2+2H2O����5��2CO2��
�����������Ҫ���ջ�ѧ����ʽ����д�����������ʵ����ʵȷ����֪ʶ��ֻ���������ܶ���ط��������������ȷ���жϣ�
��2�����ݼ���������CO2�����Ĵ�ʩ���н��
��3���������ʵ���ɫ��״̬���ܶȡ�ˮ���Եȷ�������ʲ���Ҫͨ����ѧ�仯���ֳ����������������ʽ��н��
��4�����ݼ���ȼ�����ɶ�����̼��ˮ���н��
��5�����ݻ�ѧ��Ӧǰ��ԭ�ӵ��������Ŀ������н��
����⣺��1������Ԫ�����ڱ��ṩ����Ϣ����֪̼Ԫ�ص����ԭ������Ϊ12.01��
��2��A��������Ȼ�������Դ�������ˮ�������ȼ���������Dz���ʵ�ģ�Ŀǰ�����У�
B��ʹú���ȼ�գ�Ҳͬ�������ɴ����Ķ�����̼���������������CO2������
C��ú��ʯ�͡���Ȼ���ȿ���ȼ�����ִ�������Ҫԭ�ϣ����ܽ�ֹʹ�ã��˷�Ŀǰ����ʵ��
D����������Դ�����ٶ�����̼���ŷţ����Լ�������ЧӦ������Ŀǰ���У�
��3������Ҫͨ����ѧ�仯���ֳ��������ʾ����������ʣ��������ڼ״��������ʵ��ǣ���ɫ���Դ��ƾ�ζ��Һ�塢�ܶȱ�ˮС������ˮ����ܣ�
��4������ȼ�����ɶ�����̼��ˮ����ѧ����ʽΪCH4+2O2
 CO2+2H2O��
CO2+2H2O����5���ɷ�Ӧ�Ļ�ѧ����ʽ2CaCO3+O2+2SO2�T2CaSO4+X�����ݻ�ѧ�仯ǰ��ԭ�ӵ����ࡢ��Ŀ���䣬���ж�������X�ĺ���2��Cԭ�Ӻ�4��Oԭ�ӣ���ÿ��������1��Cԭ�Ӻ�2��Oԭ�ӹ��ɣ����ʵĻ�ѧʽΪCO2��
�ʴ�Ϊ����1��12.01����2��D����3����ɫ���Դ��ƾ�ζ��Һ�塢�ܶȱ�ˮС������ˮ����ܣ���4��CH4+2O2
 CO2+2H2O����5��2CO2��
CO2+2H2O����5��2CO2�������������Ҫ���ջ�ѧ����ʽ����д�����������ʵ����ʵȷ����֪ʶ��ֻ���������ܶ���ط��������������ȷ���жϣ�

��ϰ��ϵ�д�
�����Ŀ
 ̼�������������һ��Ԫ�أ�̼�Ļ���������Ȼ������Ҫ�����ʣ�
̼�������������һ��Ԫ�أ�̼�Ļ���������Ȼ������Ҫ�����ʣ�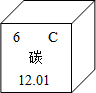 ̼�������������һ��Ԫ�أ�̼�Ļ���������Ȼ������Ҫ�����ʣ�
̼�������������һ��Ԫ�أ�̼�Ļ���������Ȼ������Ҫ�����ʣ� 2CaSO4+________��
2CaSO4+________��
 ��ԭ������Ϊ ��
��ԭ������Ϊ ��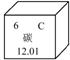 A�����ˮ�������ȼ������
A�����ˮ�������ȼ������