��Ŀ����
��������2010-2011ѧ���п���������Ѿ���ǰ��ɣ�����ش��й����⣺
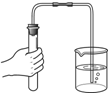
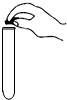
��1��������С��ͬѧ�IJ��ֲ�������ͼ��������Ǽ��ʦ���������IJ�������������������ȷ�������������ٷ֣�����Ϊ5�֣�ÿ��һ������1�֣�����______�֣�����������ֵ������У��ֲ����뾭��ĥɰ�����ǣ�______��ֻ��һ�֣�
��2���ڿ��Թ����У�С������ķ��֣������CO2�ļ���ƿ�е��������ij���ʯ��ˮʱ��ʯ��ˮ����������ˣ��������ʯ��ˮ�ֱ�����ˣ��ؼҺ�С��ͨ���������ϵ�֪��������̼��ˮ��̼�����ͨ�����Ϸ�Ӧ�ܱ��̼�������Һ���йصĻ�ѧ����ʽΪ��______��
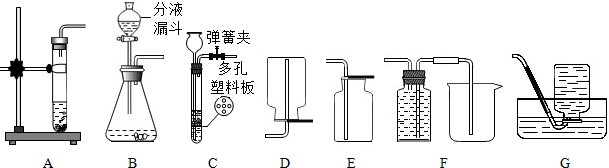
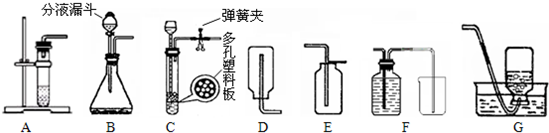
��3��������綼���ᳫ����̼���á���������ͼ��AEװ����ȡCO2��������һ���ԭ���ǣ�______��ֻ��һ�����ɣ�����֪��CO2�����ڱ��͵�̼��������Һ�����Ҫ�Ľ�CO2��ȡװ�ã�ʹ֮���ϡ���̼���á�����ѡ����ǣ�______������ͼ��ţ���
�⣺��1��װ�����ҩƷ����ʯʱ�Թ�Ӧƽ�ţ���������ȡҩƷ��Ӧ�����ӣ�ͼʾ�����й��ƿ����ҩƷ�Ĵ��棬����Ҫ�ܷ⣬��Ҫĥɰ����������ƿ�����ռ�����ʹ������壬��Ҫ�ܷ⣬�ڼ���ƿ������Χ������ĥɰ����������ʹë����Ƭ�����ܷ⣩��
��2���������⣬̼����������̼��ˮ�������Ϸ�Ӧ���ɿ�����ˮ��̼����ƣ�����ʽ��CaCO3+H2O+CO2=Ca��HCO3��2��
��3��A����װ�ò�����ʱ���Ʒ�Ӧ�ķ�������ֹ�������ڲ����˶������ſ������ռ����в��ֶ�����̼�˶��������У�������ɷ�Ӧ��ҩƷ���˷ѣ������ϡ���̼���á����CO2�����ڱ��͵�̼��������Һ�����Ҫ�Ľ�CO2��ȡװ�ã�ʹ֮���ϡ���̼���á����ɽ�����װ�ø�ΪC����ͨ�����ɼеĿ�����ʱ���Ʒ�Ӧ�ķ�����ֹͣ��ѡ��G�ռ�װ�ã���ˮ���з��뱥�͵�̼��������Һ���ܼ����жϳ����ռ��������ٶ�����̼���˷ѣ�������Լ��ҩƷ��
�ʴ�Ϊ����1��4������ƿ����ϸ��ƿ����
��2��CaCO3+H2O+CO2=Ca��HCO3��2��
��3��A�з�Ӧһ����������ֹ���˷�ҩƷ���������ɣ���CG��
��������1������ʵ�����������ע������������������ú���;���ش��⣬Ҫ������ǵ����ƣ�����ĥɰ��������ʹ�����ܷ��Ͻ���
��2���ݶ�����̼��ˮ��̼�����ͨ�����Ϸ�Ӧ�ܱ��̼�������Һ��д����ʽ��
��3��A����װ�ò�����ʱ���Ʒ�Ӧ�ķ�������ֹ�������ڲ����˶������ſ������ռ����в��ֶ�����̼�˶��������У�����˷ѣ��ݶ�����̼�����ʺ�װ���ص�ѡ����ϡ���̼���á���װ�ã�
������ͨ���ش���֪���˿ڲ�������ĥɰ����Ϊ��������ʹ���������ܷ⣬������ʹ�Լ��������ܳ������ܾݶ�����̼�����ʡ�װ���ص����������������⣮
��2���������⣬̼����������̼��ˮ�������Ϸ�Ӧ���ɿ�����ˮ��̼����ƣ�����ʽ��CaCO3+H2O+CO2=Ca��HCO3��2��
��3��A����װ�ò�����ʱ���Ʒ�Ӧ�ķ�������ֹ�������ڲ����˶������ſ������ռ����в��ֶ�����̼�˶��������У�������ɷ�Ӧ��ҩƷ���˷ѣ������ϡ���̼���á����CO2�����ڱ��͵�̼��������Һ�����Ҫ�Ľ�CO2��ȡװ�ã�ʹ֮���ϡ���̼���á����ɽ�����װ�ø�ΪC����ͨ�����ɼеĿ�����ʱ���Ʒ�Ӧ�ķ�����ֹͣ��ѡ��G�ռ�װ�ã���ˮ���з��뱥�͵�̼��������Һ���ܼ����жϳ����ռ��������ٶ�����̼���˷ѣ�������Լ��ҩƷ��
�ʴ�Ϊ����1��4������ƿ����ϸ��ƿ����
��2��CaCO3+H2O+CO2=Ca��HCO3��2��
��3��A�з�Ӧһ����������ֹ���˷�ҩƷ���������ɣ���CG��
��������1������ʵ�����������ע������������������ú���;���ش��⣬Ҫ������ǵ����ƣ�����ĥɰ��������ʹ�����ܷ��Ͻ���
��2���ݶ�����̼��ˮ��̼�����ͨ�����Ϸ�Ӧ�ܱ��̼�������Һ��д����ʽ��
��3��A����װ�ò�����ʱ���Ʒ�Ӧ�ķ�������ֹ�������ڲ����˶������ſ������ռ����в��ֶ�����̼�˶��������У�����˷ѣ��ݶ�����̼�����ʺ�װ���ص�ѡ����ϡ���̼���á���װ�ã�
������ͨ���ش���֪���˿ڲ�������ĥɰ����Ϊ��������ʹ���������ܷ⣬������ʹ�Լ��������ܳ������ܾݶ�����̼�����ʡ�װ���ص����������������⣮

��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ