��Ŀ����
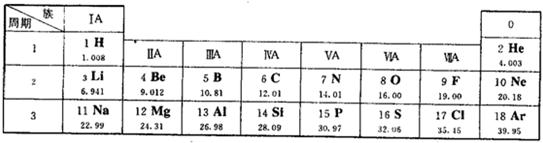
��1�����̼�����ԭ��������
��2������˵����ȷ����
A����ͬ��Ԫ�صı�����������������ͬ B��ԭ����������һ������������
C��ͬһ����������������ͬ D����ԭ�Ӻ������ӵ���������ͬ
��3������Ԫ�����ڱ������Ƕ�Ԫ�ص���ʶ����ȫ�µķ�Ծʱ�ڣ����գ�����˹��ѧ���������ǽ��˹��ϳɵ�118��Ԫ�أ����Ԫ�صĺ˵����Ϊ
��4����Ԫ�صĻ�ѧ��������������Ԫ�صĻ�ѧ��������
A��
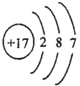 B��
B�� C��
C��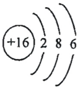
��2��������ͬԪ����������ͬ��ԭ�ӽṹ��Ԫ�����ڱ������й��ɽ��н��
��3������ԭ����������ԭ�ӵĺ˵�������н��
��4������Ԫ�صĻ�ѧ���ʸ�����ԭ�ӵ�����������Ŀ��ϵ�dz����У�������������ͬ��Ԫ�ػ�ѧ�������ƣ����н��
��2��A����ͬԪ����������ͬ����ͬ��Ԫ�صı�����������������ͬ��A˵����ȷ��B��ԭ����������һ�����ں˵��������һ��������������B˵������C��ͬһ���ڵ��Ӳ�����ͬ��C˵������D����ԭ�Ӻ������ӵ���������ͬ�������������ͬ��D˵������ͬ��
��3����118��Ԫ��ָ�ĸ�Ԫ����ԭ���������������յ�֪ʶ��֪��ԭ������=ԭ�ӵĺ˵���������Ԫ�صĺ˵����Ϊ118��
��4������Ԫ�ػ�ѧ���ʵ���������������ԭ�ӵ�������������ͬ���������ƵĻ�ѧ���ʣ����ڷ���������������A��Ϊ7���ʾ������ƵĻ�ѧ���ʣ�
�ʴ�Ϊ��
��1��12.01��A13+�� ��2��A�� ��3��118�� ��4��A��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�Ԫ�����ڱ���ѧϰ���о���ѧ����Ҫ���ߡ�������±���Ԫ�����ڱ��IJ������ݣ��ش��й����⣺
|
��
���� |
I A |
II A |
IIIA |
IVA |
VA |
VIA |
VIIA |
0 |
|
2 |
3 Li � 6��941 |
4 Be �� 9��012 |
5 B �� 10��81 |
6 C ̿ 12��01 |
7 N �� 14��01 |
8 O �� 16��00 |
9 F �� 19��00 |
10 Ne �� 20��18 |
|
3 |
11 Na �� 22��99 |
12 Mg þ 24��31 |
13 Al �� 26��98 |
14 Si �� 28��09 |
15 P �� 30��97 |
16 S �� 32��06 |
17 Cl �� 35��45 |
18 Ar � 39��95 |
��1������ϱ��в��������Ԫ�ص�һ����Ϣ��___________________________��
��2������ϱ����ҳ�һ��Ԫ�������Ǵ���ģ�����Ϊ ��
��3����3���ڣ����У������ڽ���Ԫ�ص��� ����һ��Ԫ�ط��ţ������������� ��
��4���ڽ����������У�þԪ�ر���Ԫ�ػ��ã������ԭ�ӽṹ�Ĺ�����н��� ��
��5����9�ŷ�Ԫ�غ���Ԫ���γɵĻ������ˮ��Һ����ᣨHF��,�����ڲ������, ����Ҫԭ����������벣������Ҫ�ɷֶ������裨SiO2��������Ӧ�������ķ��������壨SiF4����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
Ԫ�����ڱ���ѧϰ���о���ѧ����Ҫ���ߡ�������±���Ԫ�����ڱ��IJ������ݣ��ش��й����⣺
|
|
I A |
II A |
IIIA |
IVA |
VA |
VIA |
VIIA |
0 |
|
2 |
3 Li � 6.941 |
4 Be �� 9.012 |
5 B �� 10.81 |
6 C ̼ 12.01 |
7 N �� 14.01 |
8 O �� 16.00 |
9 F �� 19.00 |
10 Ne �� 20.18 |
|
3 |
11 Na �� 22.99 |
12 Mg þ 24.31 |
13 Al �� 26.98 |
14 Si �� 28.09 |
15 P �� 30.97 |
16 S �� 32.06 |
17 Cl �� 35.45 |
18 Ar � 39.95 |
��1������ϱ��в��������Ԫ�ص�һ����Ϣ�� ��
��2����3���������ԭ���������Ľ���Ԫ�ص��� ��
��3����Ԫ�����ڱ��У�ͬһ�壨���У���Ԫ�ؾ������ƵĻ�ѧ���ʡ������и���Ԫ�ؾ������ƻ�ѧ���ʵ��� �����ţ���
a.C��Ne b.Be��Mg c.Al��Si d.F��Cl
��4����9�ŷ�Ԫ�غ���Ԫ���γɵĻ������ˮ��Һ����ᣨHF���������ڲ�����̣�����Ҫԭ����������벣������Ҫ�ɷֶ������裨SiO2��������Ӧ�������ķ����������ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��