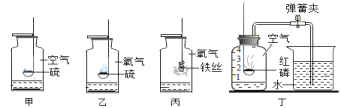
��Ŀ����
����Ŀ���Ȼ������ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
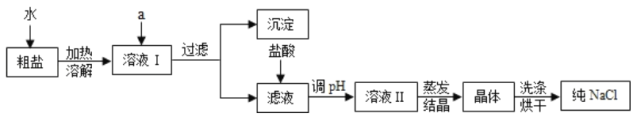
(1)���γ���NaCl�⣬����������MgCl2��CaCl2��Na2SO4�Լ���ɳ�����ʡ������Ǵ����ᴿ�IJ������̡�
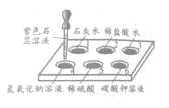
�ṩ���Լ���Na2CO3��Һ��K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba(NO3)2��Һ������NaCl��Һ��
������ȥ��Һ���е�MgCl2��CaCl2��Na2SO4�����ṩ���Լ���ѡ��a���������� �������μ�˳������Ϊ��������NaOH��Һ��������__________��Һ��������____________��Һ
������Һ�м�����������dz�ȥ__________����֤���Ἲ���� �ķ�����������_____________��
(2)���ᴿ��NaCl����200g0.9%��������ˮ����ҪNaCl������Ϊ_________��
�����Ƹ���Һʱ����Ҫ�IJ���������_________��
��������ƺ���Һ���ʵ�������������0.9%������Ϊ���ܵ�ԭ����____________��
A.����NaCl�IJ������������̷���ֽƬ������δ��
B.����Ͳ��ȡˮʱ����������ȡˮʱ���Ӷ���
C.�ձ�����ˮ
D.�Ȼ����л�������
���𰸡�BaCl2��Һ Na2CO3��Һ ��ȥ������NaOH��Na2CO3 ȡ��Һ���������μ�̼������Һ�������ݲ��� 1.8 �ձ�������������Ͳ����ͷ�ι� ACD
��������
(1)��Ҫ��ȥ��Һ���е�MgCl2��CaCl2��Na2SO4����ѡ�Լ��ֱ�������������Һ��̼������Һ���Ȼ�����Һ����������������Һ�dz�ȥMg2+�������Ȼ�����Һ�dz�ȥSO42-������̼������Һ�dz�ȥ������Ba2+����������Ҫ��Ϊ�˸��õİ����ʳ�ȥ���������µ����ʣ�������Һ��˳��������������Һ��������BaCl2��Һ��������Na2CO3��Һ��
�����ڹ��˺����Һ�к����������ƺ�̼���ƣ�����Ҫ�������������ᣬĿ���� ��ȥ������NaOH��Na2CO3����֤���Ἲ�����ķ����������ǣ�ȡ��Һ�������� �μ�̼������Һ�������ݲ�����
(2 )�Ȼ������ʵ�����=��Һ�����������ʵ���������=200g��0.9%=1.8g��
��������Һ��Ҫ�IJ��������У��ձ�������������Ͳ����ͷ�ιܣ�
��A������NaCl�IJ������������̷���ֽƬ������δ�ţ���ȡ���Ȼ���ƫС����Һ������������������0.9%����ѡ����ȷ��
B������Ͳ��ȡˮʱ����������ȡˮʱ���Ӷ�����ˮ��ʵ�����ƫС����Һ������������������0.9%����ѡ�����
C���ձ�����ˮ��ˮ����ƫ�࣬������Һ������������������0.9%����ѡ����ȷ��
D���Ȼ����л������ʣ��Ȼ��Ƶ�ʵ������ƫС����Һ������������������0.9% ����ѡ����ȷ����ѡ��ACD��

 ������ϵ�д�
������ϵ�д�����Ŀ��ˮ��һ����Ҫ������,���ճ�����ͺ�����ʵ�������Ų�����������á�
|
|
|
|
ͼ1 | ͼ2 | ͼ3 | ͼ4 |
(1)ˮ�ľ���
��ͼ1��ʾ��ˮ���ɳ�ȥˮ�е�ɫ�غ���ζ,����Ϊ���еĻ���̿����_____________�ԡ�
����ͼ1��ͼ2ʾ��ľ�ˮԭ����,�ܽ���ˮӲ�ȵľ�ˮ������ͼ______(����1����2)��
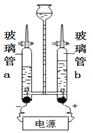
(2)ˮ����ɡ�
��ͼ3��ˮ�ĵ��װ��,��ֱͨ����Դһ��ʱ���,������b�ڲ�����������__________________,�÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________��
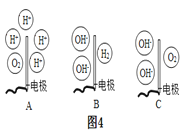
�ڵ��ˮ��ʵ����,�ڲ���������Ӻ��з�̪����������Һ,������ǿ�����ԡ���ʵ�������,�۲쵽��a�缫��������ҺѸ�ٱ��,��ô���Һ��_______(����������������������)�ԡ�����,����Һ��Ͼ��Ⱥ�����Һ��pH=7,��ͼ4���ܱ�ʾ���ʱ��b��ˮ�ڵ缫���������仯�õ���������_____(���Ӧѡ�����ĸ)��
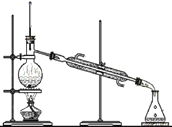
(3)ˮ�ڻ�ѧʵ���о�����Ҫ���á�
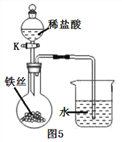
����˿���ڳ�ʪ�Ŀ�����(��ͼ5��ʾ),�ر�K,һ��ʱ���,�۲쵽������Һ������;��K,�۲쵽������Һ���½�,���ܿ������ݳ�������͵�����Һ���������½���ԭ��:_________________________��