��Ŀ����
�û�ѧ���Ż��������Ʊ�ʾ��
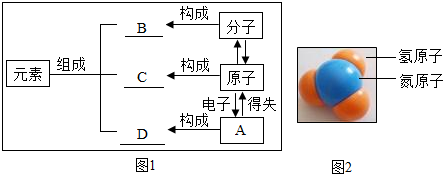
��1��2����ԭ��
��2��+2��þԪ��
��
��3����Է���������С��������
��4������������
��5��3���������
��6��3Fe2+
��7������
��8�������
��9��6Cl2O
��10��̼����
��11��2���ȷ���
��12����������
��1��2����ԭ��
2Au
2Au
����2��+2��þԪ��
| +2 |
| Mg |
| +2 |
| Mg |
��3����Է���������С��������
H2O
H2O
����4������������
2H+
2H+
����5��3���������
3H2S
3H2S
����6��3Fe2+
3����������
3����������
����7������
Ne
Ne
����8�������
��NH4��2SO4
��NH4��2SO4
����9��6Cl2O
6��һ�������ȷ���
6��һ�������ȷ���
����10��̼����
Na2CO3
Na2CO3
����11��2���ȷ���
2Cl2
2Cl2
����12����������
Al��OH��3
Al��OH��3
����������ѧ������Χ�����ֱ�ʾ��ͬ�����壺����ǰ������֣���ʾԭ�ӻ���Ӹ��������Ͻǵ����ֱ�ʾһ�����������ĵ���������½ǵ����ֱ�ʾ����ԭ�ӹ���һ�����ӣ�Ԫ�����Ϸ������ֱ�ʾԪ�صĻ��ϼۣ���Է���������С����������ˮ��һ�������Ӵ�һ����λ������ɣ�һ�������������������ԭ�Ӻ�һ����ԭ�ӹ��ɵģ�һ���������Ӵ�������λ������ɣ�����������ԭ��ֱ�ӹ��ɵģ��������笠�+1�ۣ������-2�ۣ�̼����������+1�ۣ�̼���-2�ۣ����������У�����+3�ۣ���������-1�ۣ�
����⣺��1������ǰ������֣���ʾԭ�Ӹ������ʴ�Ϊ��2Au
��2��Ԫ�����Ϸ������ֱ�ʾԪ�صĻ��ϼۣ��ʴ�Ϊ��
��3����Է���������С����������ˮ���ʴ�Ϊ��H2O
��4��һ�������Ӵ�һ����λ������ɣ��ʴ�Ϊ��2H+
��5��һ�������������������ԭ�Ӻ�һ����ԭ�ӹ��ɵģ��ʴ�Ϊ��3H2S
��6��һ���������Ӵ�������λ������ɣ��ʴ�Ϊ��3����������
��7������������ԭ��ֱ�ӹ��ɵģ��ʴ�Ϊ��Ne
��8���������笠�+1�ۣ������-2�ۣ��ʴ�Ϊ����NH4��2SO4
��9������ǰ������֣���ʾ���Ӹ������ʴ�Ϊ��6��һ�������ȷ���
��10��̼����������+1�ۣ�̼���-2�ۣ��ʴ�Ϊ��Na2CO3
��11������ǰ������֣���ʾ���Ӹ������ʴ�Ϊ��2Cl2
��12�����������У�����+3�ۣ���������-1�ۣ��ʴ�Ϊ��Al��OH��3
��2��Ԫ�����Ϸ������ֱ�ʾԪ�صĻ��ϼۣ��ʴ�Ϊ��
| +2 |
| Mg |
��3����Է���������С����������ˮ���ʴ�Ϊ��H2O
��4��һ�������Ӵ�һ����λ������ɣ��ʴ�Ϊ��2H+
��5��һ�������������������ԭ�Ӻ�һ����ԭ�ӹ��ɵģ��ʴ�Ϊ��3H2S
��6��һ���������Ӵ�������λ������ɣ��ʴ�Ϊ��3����������
��7������������ԭ��ֱ�ӹ��ɵģ��ʴ�Ϊ��Ne
��8���������笠�+1�ۣ������-2�ۣ��ʴ�Ϊ����NH4��2SO4
��9������ǰ������֣���ʾ���Ӹ������ʴ�Ϊ��6��һ�������ȷ���
��10��̼����������+1�ۣ�̼���-2�ۣ��ʴ�Ϊ��Na2CO3
��11������ǰ������֣���ʾ���Ӹ������ʴ�Ϊ��2Cl2
��12�����������У�����+3�ۣ���������-1�ۣ��ʴ�Ϊ��Al��OH��3
�����������㿼���˻�ѧʽ�����ӷ��ŵ���д��Ԫ�ط��š���ѧʽ����ѧ����ʽ�Ȼ�ѧ�������д���п�����Ҫ����֮һ��Ҫ��ǿ��ϰ������Ӧ�ã���ѧʽ��д��һ������ǣ���ǰ����Ȼ������ʮ�ֽ��淨����������Ҫ������ѡ�����������У�

��ϰ��ϵ�д�
 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
�����Ŀ