��Ŀ����
���� H2SO4 �� CuSO4 �Ļ����Һ��Ϊ�˷��������Һ�� H2SO4�� CuSO4 �������������������ͼ 1 ʵ�鷽����

ͼ1 ͼ2
��1������ͼ 1 ���꣬�Ʋ� CuSO4 ��Һ��________����ᡱ����������С��� �ԣ�C ����Һ�е�����Ϊ_______��д��ѧʽ����
��2�������û����Һ�е�CuSO4 ��������������_________��
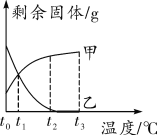
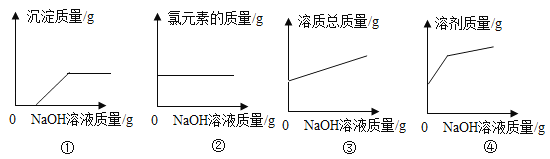
��3������100g �����Һ�в��ϼ�����������������Һ��������ͼ 2 �л�����������������Һ����������������������Ĺ�ϵͼ________��
��4�����ݸ÷�����ʵ���������֤��������������Ʒ������кͷ�Ӧ��ԭ����____��
��ϰ��ϵ�д�
�����Ŀ