��Ŀ����
��ҵ���õ���������ķ�����ȡ����������ѧ����ʽΪ��2Al2O3
4Al+3O2������⡰10t�������������������ٶ�������һ�⣬С����С����ͬѧ�ֱ���������ֲ�ͬ�ļ��㷽����
�������������Ŀ��
��1������Ϊ���ǵ�˼·�ͽ��ⷽ������ȷ��
��2����15.5g���������ȫ�ֽ���������������Ϊ���ٿˣ�����������ΪҲ�����������ַ����������Լ��㿴����
��3������Ϊ��2�����������С��ͬѧ�Ľⷨ��ֻ�з���ʲô����ʱ����ȷ��
| ||
| С���Ľ��ⷽ�� | С�ݵĽ��ⷽ�� | ||||||
| �⣺����������Ϊx 2Al2O3
204 108 10t x 204��108=10t��x x=5.3t ������������5.3t |
�⣺����������Ԫ�ص���������Ϊ��
����������10t��53%=5.3t ����������5.3t |
��1������Ϊ���ǵ�˼·�ͽ��ⷽ������ȷ��
��2����15.5g���������ȫ�ֽ���������������Ϊ���ٿˣ�����������ΪҲ�����������ַ����������Լ��㿴����
��3������Ϊ��2�����������С��ͬѧ�Ľⷨ��ֻ�з���ʲô����ʱ����ȷ��
��������1�����ݽ���IJ�����̣����������˼·�ͷ�����
��2�����ⲻ����С�ݵķ��������Ϊ���������ȫ�ֽ����Ԫ��û�ж��������У�����һ����������ء����������д��ڣ�
��3���ɣ�1���ͣ�2���Ľ�����̿�֪��С��ͬѧ�Ľⷨ���κ�����¶���ʹ�ã���ֻ���ڻ�����ֽ��Ҫ���Ԫ�ص�����û�б��ֽ����������ʱ����ʹ��С��ͬѧ�Ľⷨ��
��2�����ⲻ����С�ݵķ��������Ϊ���������ȫ�ֽ����Ԫ��û�ж��������У�����һ����������ء����������д��ڣ�
��3���ɣ�1���ͣ�2���Ľ�����̿�֪��С��ͬѧ�Ľⷨ���κ�����¶���ʹ�ã���ֻ���ڻ�����ֽ��Ҫ���Ԫ�ص�����û�б��ֽ����������ʱ����ʹ��С��ͬѧ�Ľⷨ��
����⣺��1�����ǵĽ���˼·�ͷ�������ȷ����ΪС���õ��ǡ����ݻ�ѧ����ʽ�ļ��㡱��С���õ��Ǹ���Ԫ�ص����������ļ��㣻
��2�����ⲻ����С�ݵķ��������Ϊ���������ȫ�ֽ����Ԫ��û�ж��������У�����һ����������ء����������д��ڣ�
�����15.5g���������ȫ�ֽ⣬����������������Ϊx
2KMnO4
K2MnO4+MnO2+O2��
2��158 32
15.5g x
=
��ã�x=1.6g
��3�����������غ㶨�ɣ�С��ͬѧ�Ľⷨ���κ�����¶���ʹ�ã���ֻ���ڻ�����ֽ��Ҫ���Ԫ�ص�����û�б��ֽ����������ʱ����ʹ��С��ͬѧ�Ľⷨ��
�ʴ�Ϊ����1�����ǵĽ���˼·�ͷ�������ȷ����ΪС���õ��ǡ����ݻ�ѧ����ʽ�ļ��㡱��С���õ��Ǹ���Ԫ�ص����������ļ��㣻��2�����ⲻ����С�ݵķ��������Ϊ���������ȫ�ֽ����Ԫ��û�ж��������У�����һ����������ء����������д��ڣ���������������Ϊ1.6g����3�����������غ㶨�ɣ�С��ͬѧ�Ľⷨ���κ�����¶���ʹ�ã���ֻ���ڻ�����ֽ��Ҫ���Ԫ�ص�����û�б��ֽ����������ʱ����ʹ��С��ͬѧ�Ľⷨ��
��2�����ⲻ����С�ݵķ��������Ϊ���������ȫ�ֽ����Ԫ��û�ж��������У�����һ����������ء����������д��ڣ�
�����15.5g���������ȫ�ֽ⣬����������������Ϊx
2KMnO4
| ||
2��158 32
15.5g x
| 2��158 |
| 32 |
| 15.5g |
| X |
��3�����������غ㶨�ɣ�С��ͬѧ�Ľⷨ���κ�����¶���ʹ�ã���ֻ���ڻ�����ֽ��Ҫ���Ԫ�ص�����û�б��ֽ����������ʱ����ʹ��С��ͬѧ�Ľⷨ��
�ʴ�Ϊ����1�����ǵĽ���˼·�ͷ�������ȷ����ΪС���õ��ǡ����ݻ�ѧ����ʽ�ļ��㡱��С���õ��Ǹ���Ԫ�ص����������ļ��㣻��2�����ⲻ����С�ݵķ��������Ϊ���������ȫ�ֽ����Ԫ��û�ж��������У�����һ����������ء����������д��ڣ���������������Ϊ1.6g����3�����������غ㶨�ɣ�С��ͬѧ�Ľⷨ���κ�����¶���ʹ�ã���ֻ���ڻ�����ֽ��Ҫ���Ԫ�ص�����û�б��ֽ����������ʱ����ʹ��С��ͬѧ�Ľⷨ��
������������Ҫ���鿼��ѧ��������ѧ��ѧ֪ʶ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��ҵ���õ���������ķ�����ȡ�������Ļ�ѧ����ʽΪ��2Al2O3
4Al+3O2�������10t�����������������ٶ�����С����С����λͬѧ�ֱ���������ֲ�ͬ�ļ��㷽����
����ش��������⣺
��1������Ϊ���ǵĽ���˼·�ͷ�������ȷ��
��2���ԡ�34g����������ȫ�ֽ⣨��ѧ����ʽΪ��2H2O2
2H2O+O2������������������Ϊ���ٿˣ���һ�⣬����ΪҲ�����������ַ�����������Կ���������õĽⷨ����д������
��3������Ϊ��ʲô����£�С����С��ͬѧ�Ľⷨ����ʹ�ã�
| ||
| С��ͬѧ�Ľⷨ | С��ͬѧ�Ľⷨ | |||||||||||||||||||||||||
| �⣺����������Ϊx 2Al2O3
204 108 10t x
����������5.3t���� |
�⣺����������Ԫ�ص�����������
����������lOt��53%=5.3t ����������5.3t���� |
��1������Ϊ���ǵĽ���˼·�ͷ�������ȷ��
��2���ԡ�34g����������ȫ�ֽ⣨��ѧ����ʽΪ��2H2O2
| ||
��3������Ϊ��ʲô����£�С����С��ͬѧ�Ľⷨ����ʹ�ã�
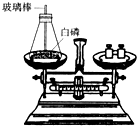 ��1����ͼ��ʾΪ�ü��Ⱥ�IJ�������ȼ���ף����ⶨ����ȼ��ǰ�������ı仯�����������֤�����غ㶨�ɵ�ʵ�飮
��1����ͼ��ʾΪ�ü��Ⱥ�IJ�������ȼ���ף����ⶨ����ȼ��ǰ�������ı仯�����������֤�����غ㶨�ɵ�ʵ�飮