��Ŀ����
 Ϊ�˼���ijʯ�����Ƿ���̼���������������ʵ�飨װ�ü�ͼ����������ƿ�м���ʯ����Ʒ��飬�ټ���һ������ϡ���ᣬ�۲�ʵ�����ó����ۣ��Իش��������⣮
Ϊ�˼���ijʯ�����Ƿ���̼���������������ʵ�飨װ�ü�ͼ����������ƿ�м���ʯ����Ʒ��飬�ټ���һ������ϡ���ᣬ�۲�ʵ�����ó����ۣ��Իش��������⣮��1���Թ���Ӧ�����������
����ʯ��ˮ
����ʯ��ˮ
����2������۲쵽��ƿ����
����
����
�������Թ��г���
����ʯ��ˮ�����
����ʯ��ˮ�����
������֤����ʯ���к���̼�������3������̼�����������̼��ƣ���д����ƿ���������Ļ�ѧ����ʽ
CaCO3+2HCl�TCaCl2+CO2��+H2O
CaCO3+2HCl�TCaCl2+CO2��+H2O
��������̼���εļ��鷽����ȡ��Ʒ�μ�ϡ���ᣬ����������ͨ������ʯ��ˮ������ʯ��ˮ����ǣ�˵����ȡ���ʺ���̼�����
��������ʵ���Ҽ���̼����ķ�������ɱ���������������⣮
��������ʵ���Ҽ���̼����ķ�������ɱ���������������⣮
����⣺��1��Ϊ������Ʒ��ϡ���ᷴӦ�����˶�����̼���壬���Թ���Ӧ�������������̼��Ӧ���ֻ��ǵij���ʯ��ˮ��
�ʴ�Ϊ������ʯ��ˮ��
��2��̼������ϡ���ᷴӦʱ���������������������̼����˿ɹ۲쵽���γ������ݣ������ԹܵĶ�����̼������ʹ����ʯ��ˮ����ǣ����ԣ����Թ��ڿɹ۲쵽�����ʯ��ˮ����ǣ�
�ʴ�Ϊ�����ݣ�����ʯ��ˮ����ǣ�
��3��̼��������ᷢ�����ֽⷴӦ�������Ȼ��ơ�ˮ�Ͷ�����̼���壻
�ʴ�Ϊ��CaCO3+2HCl�TCaCl2+CO2��+H2O��
�ʴ�Ϊ������ʯ��ˮ��
��2��̼������ϡ���ᷴӦʱ���������������������̼����˿ɹ۲쵽���γ������ݣ������ԹܵĶ�����̼������ʹ����ʯ��ˮ����ǣ����ԣ����Թ��ڿɹ۲쵽�����ʯ��ˮ����ǣ�
�ʴ�Ϊ�����ݣ�����ʯ��ˮ����ǣ�
��3��̼��������ᷢ�����ֽⷴӦ�������Ȼ��ơ�ˮ�Ͷ�����̼���壻
�ʴ�Ϊ��CaCO3+2HCl�TCaCl2+CO2��+H2O��
������ʵ����ͨ��ʹ��̼��������ϡ���ᷴӦ����������̼�ķ��������������к���̼��������ж�����̼ʹ�ó���ʯ��ˮ���飮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

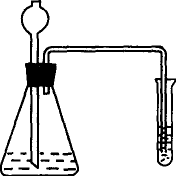
 Ϊ�˼���ijʯ�����Ƿ���̼���������������ʵ�飨װ�ü�ͼ����������ƿ�м���ʯ����Ʒ��飬�ټ���һ������ϡ���ᣬ�۲�ʵ�����ó����ۣ��Իش��������⣮
Ϊ�˼���ijʯ�����Ƿ���̼���������������ʵ�飨װ�ü�ͼ����������ƿ�м���ʯ����Ʒ��飬�ټ���һ������ϡ���ᣬ�۲�ʵ�����ó����ۣ��Իش��������⣮